Thermostability
Thermostability is the quality of a substance to resist irreversible change in its chemical or physical structure, often by resisting decomposition or polymerization, at a high relative temperature.
Thermostable materials may be used industrially as fire retardants. A thermostable plastic, an uncommon and unconventional term, is likely to refer to a thermosetting plastic that cannot be reshaped when heated, than to a thermoplastic that can be remelted and recast.
Thermostability is also a property of some proteins. To be a thermostable protein means to be resistant to changes in protein structure due to applied heat.
Thermostable proteins
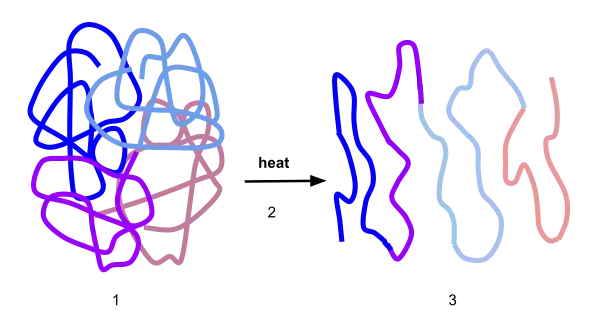
Most life-forms on Earth live at temperatures of less than 50 °C, commonly from 15 to 50 °C. Within these organisms are macromolecules (proteins and nucleic acids) which form the three-dimensional structures essential to their enzymatic activity.[2] Above the native temperature of the organism, thermal energy may cause the unfolding and denaturation, as the heat can disrupt the intramolecular bonds in the tertiary and quaternary structure. This unfolding will result in loss in enzymatic activity, which is understandably deleterious to continuing life-functions. An example of such is the denaturing of proteins in albumen from a clear, nearly colourless liquid to an opaque white, insoluble gel.
Proteins capable of withstanding such high temperatures compared to proteins that cannot, are generally from microorganisms that are hyperthermophiles. Such organisms can withstand above 50 °C temperatures as they usually live within environments of 85 °C and above.[3] Certain thermophilic life-forms exist which can withstand temperatures above this, and have corresponding adaptations to preserve protein function at these temperatures.[4] These can include altered bulk properties of the cell to stabilize all proteins,[5] and specific changes to individual proteins. Comparing homologous proteins present in these thermophiles and other organisms reveal some differences in the protein structure. One notable difference is the presence of extra hydrogen bonds in the thermophile's proteins—meaning that the protein structure is more resistant to unfolding. Similarly, thermostable proteins are rich in salt bridges or/and extra disulfide bridges stabilizing the structure.[6][7] Other factors of protein thermostability are compactness of protein structure,[8] oligomerization,[9] and strength interaction between subunits.
Uses and applications
Polymerase chain reactions
Thermostable enzymes such as Taq polymerase and Pfu DNA polymerase are used in polymerase chain reactions (PCR) where temperatures of 94 °C or over are used to melt apart DNA strands in the denaturation step of PCR.[10] This resistance to high temperature allows for DNA polymerase to elongate DNA with a desired sequence of interest with the presence of dNTP's.
Protein purification
Knowledge of an enzyme's resistance to high temperatures is especially beneficial in protein purification. In the procedure of heat denaturation, one can subject a mixture of proteins to high temperatures, which will result in the denaturation of proteins that are not thermostable, and the isolation of the protein that is thermodynamically stable. One notable example of this is found in the purification of alkaline phosphatase from the hyperthermophile Pyrococcus abyssi. This enzyme is known for being heat stable at temperatures greater than 95 °C, and therefore can be partially purified by heating when heterologously expressed in E. coli.[11] The increase in temperature causes the E. coli proteins to precipitate, while the P. abyssi alkaline phosphatase remains stably in solution.
Glycoside hydrolases
Another important group of thermostable enzymes are glycoside hydrolases. These enzymes are responsible of the degradation of the major fraction of biomass, the polysaccharides present in starch and lignocellulose. Thus, glycoside hydrolases are gaining great interest in biorefining applications in the future bioeconomy.[12] Some examples are the production of monosaccharides for food applications as well as use as carbon source for microbial conversion in fuels (ethanol) and chemical intermediates, production of oligosaccharides for prebiotic applications and production of surfactants alkyl glycoside type. All of these processes often involve thermal treatments to facilitate the polysaccharide hydrolysis, hence give thermostable variants of glycoside hydrolases an important role in this context.
Approaches to improve thermostability of proteins
Protein engineering can be used to enhance the thermostability of proteins. A number of site-directed and random mutagenesis techniques,[13][14] in addition to directed evolution,[15] have been used to increase the thermostability of target proteins. Comparative methods have been used to increase the stability of mesophilic proteins based on comparison to thermophilic homologs.[16][17][18][19] Additionally, analysis of the protein unfolding by molecular dynamics can be used to understand the process of unfolding and then design stabilizing mutations.[20] Rational protein engineering for increasing protein thermostability includes mutations which truncate loops, increase salt bridges[21] or hydrogen bonds, introduced disulfide bonds.[22] In addition, ligand binding can increase the stability of the protein, particularly when purified.[23] There are various different forces that allow for the thermostability of a particular protein. These forces include hydrophobic interactions, electrostatic interactions, and the presence of disulfide bonds. The overall amount of hydrophobicity present in a particular protein is responsible for its thermostability. Another type of force that is responsible for thermostability of a protein is the electrostatic interactions between molecules. These interactions include salt bridges and hydrogen bonds. Salt bridges are unaffected by high temperatures, therefore, are necessary for protein and enzyme stability. A third force used to increase thermostability in proteins and enzymes is the presence of disulfide bonds. They present covalent cross-linkages between the polypeptide chains. These bonds are the strongest because they’re covalent bonds, making them stronger than intermolecular forces.[24] Glycosylation is another way to improve the thermostability of proteins. Stereoelectronic effects in stabilizing interactions between carbohydrate and protein can lead to the thermostabilization of the glycosylated protein.[25]
Thermostable toxins
Certain poisonous fungi contain thermostable toxins, such as amatoxin found in the death cap and autumn skullcap mushrooms and patulin from molds. Therefore, applying heat to these will not remove the toxicity and is of particular concern for food safety.[26]
See also
- Thermophiles
References
- ↑ Kulkarni TS, Khan S, Villagomez R, Mahmood T, Lindahl S, Logan DT, Linares-Pastén JA, Nordberg Karlsson E (May 2017). "Crystal structure of β-glucosidase 1A from Thermotoga neapolitana and comparison of active site mutants for hydrolysis of flavonoid glucosides". Proteins. 85 (5): 872–884. doi:10.1002/prot.25256. PMID 28142197. S2CID 27832389.
- ↑ Kandhari, Nitika; Sinha, Somdatta (June 26, 2017). "Complex network analysis of thermostable mutants of Bacillus subtilis Lipase A". Applied Network Science. 2 (1): 18. doi:10.1007/s41109-017-0039-y. ISSN 2364-8228. PMC 6214246. PMID 30443573.
- ↑ Danson MJ, Hough DW, Russell RJ, Taylor GL, Pearl L (August 1996). "Enzyme thermostability and thermoactivity". Protein Engineering. 9 (8): 629–30. doi:10.1093/protein/9.8.629. PMID 8875639.
- ↑ Takami H, Takaki Y, Chee GJ, Nishi S, Shimamura S, Suzuki H, Matsui S, Uchiyama I (2004). "Thermoadaptation trait revealed by the genome sequence of thermophilic Geobacillus kaustophilus". Nucleic Acids Research. 32 (21): 6292–303. doi:10.1093/nar/gkh970. PMC 535678. PMID 15576355.
- ↑ Neves C, da Costa MS, Santos H (December 2005). "Compatible solutes of the hyperthermophile Palaeococcus ferrophilus: osmoadaptation and thermoadaptation in the order thermococcales". Applied and Environmental Microbiology. 71 (12): 8091–8. Bibcode:2005ApEnM..71.8091N. doi:10.1128/AEM.71.12.8091-8098.2005. PMC 1317470. PMID 16332790.
- ↑ Das R, Gerstein M (May 2000). "The stability of thermophilic proteins: a study based on comprehensive genome comparison". Functional & Integrative Genomics. 1 (1): 76–88. doi:10.1007/s101420000003. PMID 11793224. S2CID 2717885.
- ↑ Matsumura M, Becktel WJ, Levitt M, Matthews BW (September 1989). "Stabilization of phage T4 lysozyme by engineered disulfide bonds". Proceedings of the National Academy of Sciences of the United States of America. 86 (17): 6562–6. Bibcode:1989PNAS...86.6562M. doi:10.1073/pnas.86.17.6562. PMC 297884. PMID 2671995.
- ↑ Thompson MJ, Eisenberg D (July 1999). "Transproteomic evidence of a loop-deletion mechanism for enhancing protein thermostability". Journal of Molecular Biology. 290 (2): 595–604. doi:10.1006/jmbi.1999.2889. PMID 10390356.
- ↑ Tanaka Y, Tsumoto K, Yasutake Y, Umetsu M, Yao M, Fukada H, Tanaka I, Kumagai I (July 2004). "How oligomerization contributes to the thermostability of an archaeon protein. Protein L-isoaspartyl-O-methyltransferase from Sulfolobus tokodaii". The Journal of Biological Chemistry. 279 (31): 32957–67. doi:10.1074/jbc.M404405200. PMID 15169774.
- ↑ Saiki RK, Gelfand DH, Stoffel S, Scharf SJ, Higuchi R, Horn GT, Mullis KB, Erlich HA (January 1988). "Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase". Science. 239 (4839): 487–91. Bibcode:1988Sci...239..487S. doi:10.1126/science.239.4839.487. PMID 2448875.
- ↑ Zappa S, Rolland JL, Flament D, Gueguen Y, Boudrant J, Dietrich J (October 2001). "Characterization of a highly thermostable alkaline phosphatase from the euryarchaeon Pyrococcus abyssi". Applied and Environmental Microbiology. 67 (10): 4504–11. Bibcode:2001ApEnM..67.4504Z. doi:10.1128/AEM.67.10.4504-4511.2001. PMC 93196. PMID 11571149.
- ↑ Linares-Pastén, J. A.; Andersson, M; Nordberg karlsson, E (2014). "Thermostable glycoside hydrolases in biorefinery technologies". Current Biotechnology. 3 (1): 26–44. doi:10.2174/22115501113026660041.
- ↑ Sarkar CA, Dodevski I, Kenig M, Dudli S, Mohr A, Hermans E, Plückthun A (September 2008). "Directed evolution of a G protein-coupled receptor for expression, stability, and binding selectivity". Proceedings of the National Academy of Sciences of the United States of America. 105 (39): 14808–13. Bibcode:2008PNAS..10514808S. doi:10.1073/pnas.0803103105. PMC 2567449. PMID 18812512.
- ↑ Asial I, Cheng YX, Engman H, Dollhopf M, Wu B, Nordlund P, Cornvik T (2013). "Engineering protein thermostability using a generic activity-independent biophysical screen inside the cell". Nature Communications. 4: 2901. Bibcode:2013NatCo...4.2901A. doi:10.1038/ncomms3901. PMID 24352381.
- ↑ Hoseki J, Yano T, Koyama Y, Kuramitsu S, Kagamiyama H (November 1999). "Directed evolution of thermostable kanamycin-resistance gene: a convenient selection marker for Thermus thermophilus". Journal of Biochemistry. 126 (5): 951–6. doi:10.1093/oxfordjournals.jbchem.a022539. PMID 10544290.
- ↑ Sayed A, Ghazy MA, Ferreira AJ, Setubal JC, Chambergo FS, Ouf A, Adel M, Dawe AS, Archer JA, Bajic VB, Siam R, El-Dorry H (January 2014). "A novel mercuric reductase from the unique deep brine environment of Atlantis II in the Red Sea". The Journal of Biological Chemistry. 289 (3): 1675–87. doi:10.1074/jbc.M113.493429. PMC 3894346. PMID 24280218.
- ↑ Perl D, Mueller U, Heinemann U, Schmid FX (May 2000). "Two exposed amino acid residues confer thermostability on a cold shock protein". Nature Structural Biology. 7 (5): 380–3. doi:10.1038/75151. PMID 10802734. S2CID 21850845.
- ↑ Lehmann M, Pasamontes L, Lassen SF, Wyss M (December 2000). "The consensus concept for thermostability engineering of proteins". Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology. 1543 (2): 408–415. doi:10.1016/s0167-4838(00)00238-7. PMID 11150616.
- ↑ Sauer DB, Karpowich NK, Song JM, Wang DN (October 2015). "Rapid Bioinformatic Identification of Thermostabilizing Mutations". Biophysical Journal. 109 (7): 1420–8. Bibcode:2015BpJ...109.1420S. doi:10.1016/j.bpj.2015.07.026. PMC 4601007. PMID 26445442.
- ↑ Liu HL, Wang WC (January 2003). "Protein engineering to improve the thermostability of glucoamylase from Aspergillus awamori based on molecular dynamics simulations". Protein Engineering. 16 (1): 19–25. doi:10.1093/proeng/gzg007. PMID 12646689.
- ↑ Lee CW, Wang HJ, Hwang JK, Tseng CP (2014). "Protein thermal stability enhancement by designing salt bridges: a combined computational and experimental study". PLOS ONE. 9 (11): e112751. Bibcode:2014PLoSO...9k2751L. doi:10.1371/journal.pone.0112751. PMC 4231051. PMID 25393107.
- ↑ Mansfeld J, Vriend G, Dijkstra BW, Veltman OR, Van den Burg B, Venema G, Ulbrich-Hofmann R, Eijsink VG (April 1997). "Extreme stabilization of a thermolysin-like protease by an engineered disulfide bond". The Journal of Biological Chemistry. 272 (17): 11152–6. doi:10.1074/jbc.272.17.11152. PMID 9111013.
- ↑ Mancusso R, Karpowich NK, Czyzewski BK, Wang DN (December 2011). "Simple screening method for improving membrane protein thermostability". Methods. 55 (4): 324–9. doi:10.1016/j.ymeth.2011.07.008. PMC 3220791. PMID 21840396.
- ↑ Tigerström, Anna (2005). "Thermostability of Proteins". BIOS. 76 (1): 22–27. doi:10.1893/0005-3155(2005)076[0022:TBFTOP]2.0.CO;2. JSTOR 4608725.
- ↑ Ardejani, Maziar S.; Noodleman, Louis; Powers, Evan T.; Kelly, Jeffery W. (2021-03-15). "Stereoelectronic effects in stabilizing protein– N -glycan interactions revealed by experiment and machine learning". Nature Chemistry. 13 (5): 480–487. Bibcode:2021NatCh..13..480A. doi:10.1038/s41557-021-00646-w. ISSN 1755-4349. PMC 8102341. PMID 33723379.
- ↑ "FDA: Moldy applesauce repackaged by school lunch supplier". NBC News. NBC News. Retrieved 15 April 2015.
External links
| Look up thermostability in Wiktionary, the free dictionary. |