Thermus aquaticus
| Thermus aquaticus | |
|---|---|
 | |
| Scientific classification | |
| Domain: | |
| Phylum: | |
| Class: | |
| Order: | |
| Family: | |
| Genus: | |
| Species: | T. aquaticus |
| Binomial name | |
| Thermus aquaticus Brock & Freeze, 1969 | |
Thermus aquaticus is a species of bacterium that can tolerate high temperatures, one of several thermophilic bacteria that belong to the Deinococcota phylum. It is the source of the heat-resistant enzyme Taq DNA polymerase, one of the most important enzymes in molecular biology because of its use in the polymerase chain reaction (PCR) DNA amplification technique.
History

When studies of biological organisms in hot springs began in the 1960s, scientists thought that the life of thermophilic bacteria could not be sustained in temperatures above about 55 °C (131 °F).[1] Soon, however, it was discovered that many bacteria in different springs not only survived, but also thrived in higher temperatures. In 1969, Thomas D. Brock and Hudson Freeze of Indiana University reported a new species of thermophilic bacteria which they named Thermus aquaticus.[2] The bacterium was first isolated from Mushroom Spring in the Lower Geyser Basin of Yellowstone National Park, which is near the major Great Fountain Geyser and White Dome Geyser,[3] and has since been found in similar thermal habitats around the world.
Biology
T. aquaticus shows best growth at 65–70 °C (149–158 °F), but can survive at temperatures of 50–80 °C (122–176 °F). It primarily scavenges for protein from its environment as is evidenced by the large number of extracellular and intracellular proteases and peptidases as well as transport proteins for amino acids and oligopeptides across its cell membrane. This bacterium is a chemotroph—it performs chemosynthesis to obtain food. However, since its range of temperature overlaps somewhat with that of the photosynthetic cyanobacteria that share its ideal environment, it is sometimes found living jointly with its neighbors, obtaining energy for growth from their photosynthesis. T. aquaticus normally respires aerobically but one of its strains, Thermus aquaticus Y51MC23, is able to be grown anaerobically.[4]
The genetic material of T. aquaticus consists of one chromosome and four plasmids, and its complete genome sequencing revealed CRISPR genes at numerous loci.[5]
Morphology
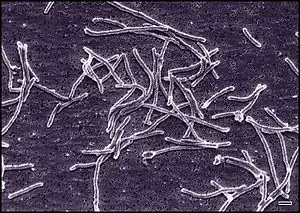
Thermus aquaticus is generally of cylindrical shape with a diameter of 0.5 μm to 0.8 μm. The shorter rod shape has a length of 5 μm to 10 μm. The longer filament shape has a length that varies greatly and in some cases exceeds 200 μm. T. aquaticus has shown multiple possible morphologies in different cultures. The rod-shaped bacteria have a tendency to aggregate. Associations of several individuals can lead to the formation of spherical bodies 10 μm to 20 μm in diameter, also called rotund bodies.[2][6] These bodies are not composed of cell envelope or outer membrane components as previously thought, but are instead made from remodelled peptidoglycan cell wall. Their exact function in the survival of T. aquaticus remains unknown but has been theorised to include temporary food and nucleotide storage, or they may play a role in the attachment and organisation of colonies.[5]
Enzymes from T. aquaticus
T. aquaticus has become famous as a source of thermostable enzymes, particularly the Taq DNA polymerase, as described below.
Aldolase
Studies of this extreme thermophilic bacterium that could be grown in cell culture was initially centered on attempts to understand how enzymes, which are normally inactive at high temperature, can function at high temperature in thermophiles. In 1970, Freeze and Brock published an article describing a thermostable aldolase enzyme from T. aquaticus.[7]
RNA polymerase
The first polymerase enzyme isolated from T. aquaticus in 1974 was a DNA-dependent RNA polymerase,[8] used in the process of transcription.
Taq I restriction enzyme
Most molecular biologists probably became aware of T. aquaticus in the late 1970s or early 1980s because of the isolation of useful restriction endonucleases from this organism.[9] Use of the term Taq to refer to Thermus aquaticus arose at this time from the convention of giving restriction enzymes short names, such as Sal and Hin, derived from the genus and species of the source organisms.
DNA polymerase ("Taq pol")
DNA polymerase was first isolated from T. aquaticus in 1976.[10] The first advantage found for this thermostable (temperature optimum 72°C, does not denature even in 95 °C) DNA polymerase was that it could be isolated in a purer form (free of other enzyme contaminants) than could the DNA polymerase from other sources. Later, Kary Mullis and other investigators at Cetus Corporation discovered this enzyme could be used in the polymerase chain reaction (PCR) process for amplifying short segments of DNA,[11] eliminating the need to add E. coli polymerase enzymes after every cycle of thermal denaturation of the DNA. The enzyme was also cloned, sequenced, modified (to produce the shorter 'Stoffel fragment'), and produced in large quantities for commercial sale.[12] In 1989 Science magazine named Taq polymerase as its first "Molecule of the Year".[13] In 1993, Mullis was awarded the Nobel Prize in Chemistry for his work with PCR.[14]
Other enzymes
The high optimum temperature for T. aquaticus allows researchers to study reactions under conditions for which other enzymes lose activity. Other enzymes isolated from this organism include DNA ligase, alkaline phosphatase, NADH oxidase, isocitrate dehydrogenase, amylomaltase, and fructose 1,6-disphosphate-dependent L-lactate dehydrogenase.
Controversy
The commercial use of enzymes from T. aquaticus has not been without controversy. After Brock's studies, samples of the organism were deposited in the American Type Culture Collection, a public repository. Other scientists, including those at Cetus, obtained it from there. As the commercial potential of Taq polymerase became apparent in the 1990s,[15] the National Park Service labeled its use as the "Great Taq Rip-off".[16] Researchers working in National Parks are now required to sign "benefits sharing" agreements that would send a portion of later profits back to the Park Service.
See also
- Biotechnology
- Geyser
- Thermophiles
References
- ↑ Thomas Brock's essay "Life at High Temperatures"
- 1 2 Brock TD; Freeze H (1969). "Thermus aquaticus, a Nonsporulating Extreme Thermophile". J. Bacteriol. 98 (1): 289–97. doi:10.1128/jb.98.1.289-297.1969. PMC 249935. PMID 5781580.
- ↑ Bryan, T. Scott (2008). Geysers of Yellowstone, The (4th ed.). University Press of Colorado. ISBN 978-0-87081-924-7.
- ↑ Pierson, Beverly K.; Bauld, John; Castenholz, Richard W.; D'Amelio, Elisa; Marais, David J. Des; Farmer, Jack D.; Grotzinger, John P.; Jørgensen, Bo Barker; Nelson, Douglas C. (1992-06-26), Schopf, J. William; Klein, Cornelis (eds.), "Modern Mat-Building Microbial Communities: a Key to the Interpretation of Proterozoic Stromatolitic Communities", The Proterozoic Biosphere (1 ed.), Cambridge University Press, pp. 245–342, doi:10.1017/cbo9780511601064.008, ISBN 978-0-521-36615-1
- 1 2 Brumm, Phillip J.; Monsma, Scott; Keough, Brendan; Jasinovica, Svetlana; Ferguson, Erin; Schoenfeld, Thomas; Lodes, Michael; Mead, David A. (2015). "Complete Genome Sequence of Thermus aquaticus Y51MC23". PLOS ONE. 10 (10): e0138674. Bibcode:2015PLoSO..1038674B. doi:10.1371/journal.pone.0138674. ISSN 1932-6203. PMC 4605624. PMID 26465632.
- ↑ Brock TD; Edwards MR (1970). "Fine Structure of Thermus aquaticus, an Extreme Thermophile". J. Bacteriol. 104 (1): 509–517. doi:10.1128/jb.104.1.509-517.1970. PMC 248237. PMID 5473907.
- ↑ Freeze H; Brock TD (1970). "Thermostable Aldolase from Thermus aquaticus". J. Bacteriol. 101 (2): 541–50. doi:10.1128/jb.101.2.541-550.1970. PMC 284939. PMID 4984076.
- ↑ Air GM; Harris JI (1974). "DNA-Dependent RNA Polymerase From the Thermophilic Bacterium Thermus aquaticus". FEBS Letters. 38 (3): 277–281. doi:10.1016/0014-5793(74)80072-4. PMID 4604362. S2CID 38553550.
- ↑ Sato, S (February 1978). "A single cleavage of Simian virus 40 (SV40) DNA by a site specific endonuclease from Thermus aquaticus, Taq I". J. Biochem. 83 (2): 633–5. doi:10.1093/oxfordjournals.jbchem.a131952. PMID 204628.
- ↑ Chien, A; Edgar DB; Trela JM (September 1, 1976). "Deoxyribonucleic acid polymerase from the extreme thermophile Thermus aquaticus". J. Bacteriol. 127 (3): 1550–7. doi:10.1128/jb.127.3.1550-1557.1976. PMC 232952. PMID 8432.
- ↑ Saiki, RK; et al. (1988). "Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase". Science. 239 (4839): 487–91. Bibcode:1988Sci...239..487S. doi:10.1126/science.239.4839.487. PMID 2448875.
- ↑ Lawyer FC; et al. (1993). "High-level expression, purification, and enzymatic characterization of full-length Thermus aquaticus DNA polymerase". Genome Research. 2 (4): 275–87. doi:10.1101/gr.2.4.275. PMID 8324500.
- ↑ Guyer RL; Koshland DE (December 1989). "The Molecule of the Year". Science. 246 (4937): 1543–6. doi:10.1126/science.2688087. PMID 2688087.
- ↑ Mullis, Kary B. (December 8, 1993). "Nobel Lecture – The Polymerase Chain Reaction".
- ↑ Fore J; Wiechers IR; Cook-Deegan R (2006). "The effects of business practices, licensing, and intellectual property on development and dissemination of the polymerase chain reaction: case study". J Biomed Discov Collab. 1: 7. doi:10.1186/1747-5333-1-7. PMC 1523369. PMID 16817955. — Detailed history of Cetus and the commercial aspects of PCR.
- ↑ Robbins J (28 November 2006). "The Search for Private Profit in the Nation's Public Parks". The New York Times.
Further reading
- Brock, Thomas D. (August 1, 1997). "The Value of Basic Research: Discovery of Thermus aquaticus and Other Extreme Thermophiles". Genetics. 146 (4): 1207–10. doi:10.1093/genetics/146.4.1207. PMC 1208068. PMID 9258667.
- Hogan, C.Michael (2010). "Extremophiles". Encyclopedia of Earth. 146 (4): 1207–10. doi:10.1093/genetics/146.4.1207. PMC 1208068. PMID 9258667.