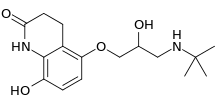
Hydroxycarteolol
 | |
| Clinical data | |
|---|---|
| Other names | 8-Hydroxycarteolol |
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C16H24N2O4 |
| Molar mass | 308.378 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Hydroxycarteolol is a beta blocker and metabolite of carteolol.[1][2]
References
- ↑ Igarashi H, Katsuta Y, Sawa K, Nakazato Y, Kawasaki T (Apr 1990). "A comparison of the opacifying effects of carteolol.HCl and 8-hydroxycarteolol.HCl in the isolated porcine cornea". Fundam Appl Toxicol. 14 (3): 554–559. doi:10.1093/toxsci/14.3.554. PMID 2340982.
- ↑ Jasper JR, Michel MC, Insel PA (1990). "The beta-adrenoceptor antagonist carteolol and its metabolite 8-hydroxycarteolol have different intrinsic sympathomimetic activities". Br J Clin Pharmacol. 30 (Suppl 1): 109S–111S. doi:10.1111/j.1365-2125.1990.tb05477.x. PMC 1368107. PMID 2176514.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.