Ideonella sakaiensis
| Ideonella sakaiensis | |
|---|---|
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | Proteobacteria |
| Class: | Betaproteobacteria |
| Order: | Burkholderiales |
| Family: | Comamonadaceae |
| Genus: | Ideonella |
| Species: | I. sakaiensis |
| Binomial name | |
| Ideonella sakaiensis Yoshida et al. 2016[1] | |
Ideonella sakaiensis is a bacterium from the genus Ideonella and family Comamonadaceae capable of breaking down and consuming the plastic polyethylene terephthalate (PET) as a sole carbon and energy source. The bacterium was originally isolated from a sediment sample taken outside of a plastic bottle recycling facility in Sakai, Japan.[2]
Discovery
Ideonella sakaiensis was first identified in 2016 by a team of researchers led by Kohei Oda of Kyoto Institute of Technology and Kenji Miyamoto of Keio University after collecting a sample of PET-contaminated sediment near a plastic bottle recycling facility in Japan.[2] The bacterium was isolated from a consortium of microorganisms in the sediment sample, including protozoa and yeast-like cells. The entire microbial community was shown to mineralize 75% of the degraded PET into carbon dioxide once it had been initially degraded and assimilated by Ideonella sakaiensis.[2]
Characterization
Ideonella sakaiensis is Gram-negative, aerobic, and rod-shaped. It does not form spores. Cells are motile and have a single flagellum. I. sakaiensis also tests positive for oxidase and catalase. The bacterium grows at a pH range of 5.5 to 9.0 (optimally at 7 to 7.5) and a temperature of 15–42 °C (optimally at 30–37 °C). Colonies of I. sakaiensis are colorless, smooth, and circular. Its size varies from 0.6 to 0.8 μm in width and 1.2-1.5 μm in length.[3] The bacterium was shown to grow on PET surfaces in a community with other I. sakaiensis cells by adhering to the PET and other cells with thin appendages. These appendages may also function to secrete PET-degrading enzymes onto the PET surface.[2]
Through phylogenetic analysis, the species was shown to be a part of the genus Ideonella, but possessed a significantly different genome than other known species in the genus, including Ideonella dechloratans and Ideonella azotifigens, thus justifying its classification as a new species.[3]
Degradation and assimilation of PET
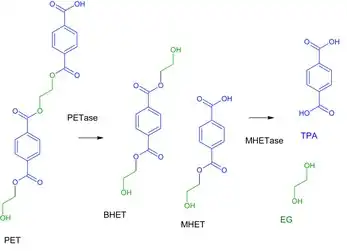
Ideonella sakaiensis PET surface and use a secreted PET hydrolase, or PETase, to degrade the PET into mono(2-hydroxyethyl)terephthalic acid (MHET), a heterodimer composed of terephthalic acid (TPA) and ethylene glycol. The PETase also degrades PET into another intermediate known as Bis-(2-hydroxyethyl) terephthalate (BHET), BHET can be converted into MHET after PET hydrolysis.[4] The I. sakaiensis PETase functions by hydrolyzing the ester bonds present in PET with high specificity. The resulting MHET is then degraded into its two monomeric constituents by a lipid-anchored MHET hydrolase enzyme, or MHETase, on the cell's outer membrane.[2] The overall mechanism of the PET plastic being broken down is exhibited in the image to the right. The monomeric constituents such as ethylene glycol is then taken up and used by I. sakaiensis and many other bacteria.[2][5] The other constituent; terephthalic acid, a more recalcitrant compound, is imported into the I. sakaiensis cell via the terephthalic acid transporter protein. Once in the cell, the aromatic terephthalic acid molecule is oxidized by terephthalic acid-1,2-dioxygenase and 1,2-dihydroxy-3,5-cyclohexadiene-1,4-dicarboxylate dehydrogenase into a catechol intermediate. The catechol ring is then cleaved by PCA 3,4-dioxygenase before the compound is integrated into other metabolic pathways (e.g. TCA cycle).[2] As a result, both of the molecules derived from the PET are used by the cell to produce energy and to build necessary biomolecules. Eventually, the assimilated carbon may be mineralized to carbon dioxide and released into the atmosphere.[2]
Impact and applications
The discovery of Ideonella sakaiensis has potential importance for the degradation of PET plastics. Prior to its discovery, the only known degraders of PET were a small number of bacteria and fungi, including Fusarium solani, and no organisms were definitively known to degrade PET as a primary carbon and energy source.[2] The discovery of I. sakaiensis spurred discussion about PET biodegradation as a method of recycling and bioremediation.[2]
The wild-type bacterium is able to colonize and break down a thin (0.2 mm thickness) film of low-crystallinity (soft) PET in approximately 6 weeks, and the responsible PETase enzyme was shown to degrade high-crystallinity (hard) PET approximately 30-fold slower (180 weeks or more than 3 years) than low-crystallinity PET.[2] A large amount of manufactured PET is highly crystalline (e.g. plastic bottles), so it is thought that any prospective applications of the I. sakaiensis PETase enzyme in recycling programs will need to be preceded by genetic optimization of the enzyme.[2][6] The MHETase enzyme could also be optimized and used in recycling or bioremediation applications in combination with the PETase enzyme. It degrades the MHET produced by the PETase into ethylene glycol and terephthalic acid.[2] Once formed, these two compounds can be further biodegraded into carbon dioxide by I. sakaiensis or other microbes, or they can be purified and used to manufacture new PET in an industrial recycling plant setting.[2][7]
Genetic engineering
The PET degrading enzyme of Ideonella sakaiensis, PETase, has been genetically modified and combined with MHETase to break down PET faster, which also degrades PEF. This along with other approaches may be useful in various efforts such as; recycling and upcycling of mixed plastics.[8][9][10]
Coagulation Filtration System
In 2021, fifth graders Julia Stewart and Jacob Park created the concept of a Coagulation Filtration System for Toshiba's ExploraVision contest, which utilizes Ideonella sakaiensis in a process that filters, coagulates, flocculates, and sediments water in a more environmentally friendly and efficient way.[11][12][13] This project won the 4-6 division of ExploraVision nationally.[11][12]
See also
- Organisms breaking down plastic
- PET bottle recycling
- PETase, the enzyme produced by this bacterium.
- Pestalotiopsis microspora, an endophytic fungus species capable of breaking down polyurethane.
References
- ↑ Yoshida, S.; Hiraga, K.; Takehana, T.; Taniguchi, I.; Yamaji, H.; Maeda, Y.; Toyohara, K.; Miyamoto, K.; Kimura, Y.; Oda, K. (10 March 2016). "A bacterium that degrades and assimilates poly(ethylene terephthalate)". Science. 351 (6278): 1196–1199. Bibcode:2016Sci...351.1196Y. doi:10.1126/science.aad6359. PMID 26965627. S2CID 31146235.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Yoshida, Shosuke; Hiraga, Kazumi; Takehana, Toshihiko; Taniguchi, Ikuo; Yamaji, Hironao; Maeda, Yasuhito; Toyohara, Kiyotsuna; Miyamoto, Kenji; Kimura, Yoshiharu (11 March 2016). "A bacterium that degrades and assimilates poly(ethylene terephthalate)". Science. 351 (6278): 1196–1199. Bibcode:2016Sci...351.1196Y. doi:10.1126/science.aad6359. ISSN 1095-9203. PMID 26965627. S2CID 31146235. Lay summary (PDF) (30 March 2016).
{{cite journal}}: Cite uses deprecated parameter|lay-date=(help) - 1 2 Yoshida, Shosuke; Hiraga, Kazumi; Takehana, Toshihiko; Taniguchi, Ikuo; Yamaji, Hironao; Maeda, Yasuhito; Toyohara, Kiyotsuna; Miyamoto, Kenji; Kimura, Yoshiharu; Oda, Kohei (11 March 2016). "A bacterium that degrades and assimilates poly(ethylene terephthalate)". Science. 351 (6278): 1196–1199. doi:10.1126/science.aad6359. ISSN 1095-9203. PMID 26965627.
- ↑ Puspitasari, Nathania; Tsai, Shen-Long; Lee, Cheng-Kang (15 April 2021). "Class I hydrophobins pretreatment stimulates PETase for monomers recycling of waste PETs". International Journal of Biological Macromolecules. 176: 157–164. doi:10.1016/j.ijbiomac.2021.02.026. ISSN 0141-8130.
- ↑ Pearce, B. A.; Heydeman, M. T. (1 May 1980). "Metabolism of Di(ethylene glycol) [2-(2'-Hydroxyethoxy)ethanol] and Other Short Poly(ethylene glycol)s by Gram-negative Bacteria". Microbiology. 118 (1): 21–27. doi:10.1099/00221287-118-1-21. ISSN 1350-0872.
- ↑ Coghlan, Andy. "Bacteria found to eat PET plastics could help do the recycling". New Scientist. Retrieved 18 March 2016.
- ↑ Al-Sabagh, A.M.; Yehia, F.Z.; Eshaq, Gh.; Rabie, A.M.; ElMetwally, A.E. (March 2016). "Greener routes for recycling of polyethylene terephthalate". Egyptian Journal of Petroleum. 25 (1): 53–64. doi:10.1016/j.ejpe.2015.03.001.
- ↑ Carrington, Damian (28 September 2020). "New super-enzyme eats plastic bottles six times faster". The Guardian. Retrieved 12 October 2020.
- ↑ "Plastic-eating enzyme 'cocktail' heralds new hope for plastic waste". phys.org. Retrieved 12 October 2020.
- ↑ Knott, Brandon C.; Erickson, Erika; Allen, Mark D.; Gado, Japheth E.; Graham, Rosie; Kearns, Fiona L.; Pardo, Isabel; Topuzlu, Ece; Anderson, Jared J.; Austin, Harry P.; Dominick, Graham; Johnson, Christopher W.; Rorrer, Nicholas A.; Szostkiewicz, Caralyn J.; Copié, Valérie; Payne, Christina M.; Woodcock, H. Lee; Donohoe, Bryon S.; Beckham, Gregg T.; McGeehan, John E. (24 September 2020). "Characterization and engineering of a two-enzyme system for plastics depolymerization". Proceedings of the National Academy of Sciences. 117 (41): 25476–25485. doi:10.1073/pnas.2006753117. ISSN 0027-8424. PMC 7568301. PMID 32989159.
 Text and images are available under a Creative Commons Attribution 4.0 International License.
Text and images are available under a Creative Commons Attribution 4.0 International License. - 1 2 "Eight Student Teams Named National Winners of 29th Annual ExploraVision Challenge". news.toshiba.com. Retrieved 3 December 2021.
- 1 2 "Loading site please wait..." www.exploravision.org. Retrieved 3 December 2021.
- ↑ "Home | Coagulation Filtration System". ExploraVision.PPT te. Retrieved 3 December 2021.