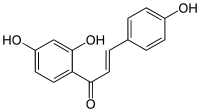
Isoliquiritigenin
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2′,4,4′-Trihydroxychalcone | |
Other names
| |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| Abbreviations | ILTG |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.202.617 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C15H12O4 |
| Molar mass | 256.257 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Isoliquiritigenin is a phenolic chemical compound found in licorice.[1]
Metabolism
The enzyme 6'-deoxychalcone synthase uses malonyl-CoA, 4-coumaroyl-CoA, NADPH, and H+ to produce CoA, isoliquiritigenin, CO2, NADP+, and H2O.
The enzyme isoliquiritigenin 2'-O-methyltransferase further transforms isoliquiritigenin into 2'-O-methylisoliquiritigenin.
Mechanism of action
Isoliquiritigenin has been found to be a potent (65 times higher affinity than diazepam) GABA-A benzodiapine receptor positive allosteric modulator.[2] It can target miR-301b/LRIG1 signaling pathways, resulting in the inhibition of melanoma growth in vitro.[3]
References
- ↑ Nerya, Ohad; Vaya, Jacob; Musa, Ramadan; Izrael, Sarit; Ben-Arie, Ruth; Tamir, Snait (2003). "Glabrene and Isoliquiritigenin as Tyrosinase Inhibitors from Licorice Roots". Journal of Agricultural and Food Chemistry. 51 (5): 1201–1207. doi:10.1021/jf020935u. PMID 12590456.
- ↑ Cho, S; Kim, S; Jin, Z; Yang, H; Han, D; Baek, N. I.; Jo, J; Cho, C. W.; Park, J. H.; Shimizu, M; Jin, Y. H. (2011). "Isoliquiritigenin, a chalcone compound, is a positive allosteric modulator of GABAA receptors and shows hypnotic effects". Biochemical and Biophysical Research Communications. 413 (4): 637–42. doi:10.1016/j.bbrc.2011.09.026. PMID 21945440.
- ↑ Xiang, Shijian; Chen, Huoji; Luo, Xiaojun; An, Baichao; Wu, Wenfeng; Cao, Siwei; Ruan, Shifa; Wang, Zhuxian; Weng, Lidong; Zhu, Hongxia; Liu, Qiang (2018). "Isoliquiritigenin suppresses human melanoma growth by targeting miR-301b/LRIG1 signaling". Journal of Experimental & Clinical Cancer Research. 37 (1): 184. doi:10.1186/s13046-018-0844-x. PMC 6091185. PMID 30081934.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.