Kurkinorin
 | |
| Names | |
|---|---|
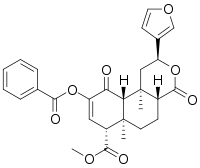
| IUPAC name
Methyl (2S,4aR,6aR,7R,10aR,10bR)-9-benzoyloxy-2-(furan-3-yl)-6a,10b-dimethyl-4,10-dioxo-2,4a,5,6,7,10a-hexahydro-1H-benzo[f]isochromene-7-carboxylate | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
PubChem CID |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C28H28O8 |
| Molar mass | 492.524 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Kurkinorin is a non-nitrogenous, extremely selective centrally acting μ-opioid receptor agonist derived from salvinorin A[1] with no sedating or rewarding effects.[2]
See also
References
- ↑ Crowley RS, Riley AP, Alder AF, Anderson RJ, Luo D, Kaska S, Maynez P, Kivell BM, Prisinzano TE (June 2020). "Synthetic Studies of Neoclerodane Diterpenes from Salvia divinorum: Design, Synthesis, and Evaluation of Analogues with Improved Potency and G-protein Activation Bias at the μ-Opioid Receptor". ACS Chemical Neuroscience. 11 (12): 1781–1790. doi:10.1021/acschemneuro.0c00191. PMC 7359744. PMID 32383854.
- ↑ Shivaperumal, Nirajmohan (May 18, 2017). Investigating the analgesic properties of Kurkinorin, a novel mu-opioid receptor analogue of Salvinorin A (Masters). Victoria University of Wellington.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.