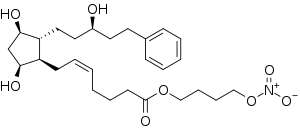
Latanoprostene bunod
 | |
| Names | |
|---|---|
| Trade names | Vyzulta |
| Other names | BOL-303259-X |
IUPAC name
| |
| Clinical data | |
| Drug class | Prostaglandin[1] |
| Main uses | Increased intraocular pressure[1] |
| Side effects | Eye pain, eye redness[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| Legal status |
|
| Chemical and physical data | |
| Formula | C27H41NO8 |
| Molar mass | 507.624 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Latanoprostene bunod, sold under the brand name Vyzulta, is a medication used to treat increased intraocular pressure in open-angle glaucoma and ocular hypertension.[1] It is used as an eye drop.
Common side effects include eye pain and eye redness.[1] Other side effects may include bacterial keratitis, macular edema, darkening of the skin around the eye, and eyelash changes.[1] It is a prostaglandin which increases outflow through the trabecular meshwork and uveoscleral pathway.[1][2]
Latanoprostene bunod was approved for medical use in the United States in 2017.[1] In the United States it costs about 230 USD for a 2.5 ml bottle as of 2021.[3] As of 2021 it is not commercially available in the United Kingdom or Europe.[4]
References
- 1 2 3 4 5 6 7 8 "Latanoprostene Bunod Monograph for Professionals". Drugs.com. Archived from the original on 4 March 2021. Retrieved 21 November 2021.
- ↑ Hoy SM (May 2018). "Latanoprostene Bunod Ophthalmic Solution 0.024%: A Review in Open-Angle Glaucoma and Ocular Hypertension". Drugs. 78 (7): 773–780. doi:10.1007/s40265-018-0914-6. PMC 5976683. PMID 29761382.
- ↑ "Vyzulta Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 16 May 2021. Retrieved 21 November 2021.
- ↑ "Latanoprostene bunod". SPS - Specialist Pharmacy Service. 25 January 2016. Archived from the original on 21 November 2021. Retrieved 21 November 2021.
External links
| Identifiers: |
|---|
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.