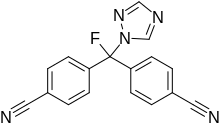
Leflutrozole
 | |
| Clinical data | |
|---|---|
| Other names | BGS-649; CGP-47645 |
| Routes of administration | By mouth |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C17H10FN5 |
| Molar mass | 303.300 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Leflutrozole (developmental code names BGS-649, CGP-47645) is an aromatase inhibitor which is under development by Mereo BioPharma and Novartis for the treatment of hypogonadism in men.[1] It was also under investigation for the treatment of endometriosis, but development for this indication was discontinued.[1] As of December 2017, leflutrozole is in phase II clinical trials for hypogonadism.[1]
See also
References
External links
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.