Lens (vertebrate anatomy)
| Lens | |
|---|---|
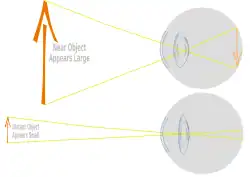 Lens of eye changing shape to focus near and far. | |
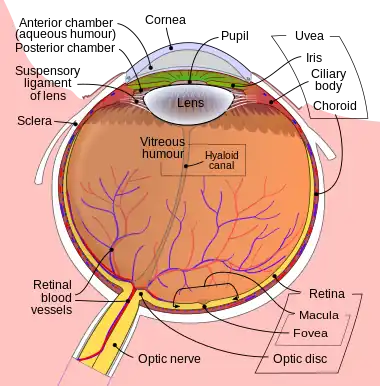 Schematic diagram of the human eye. | |
| Details | |
| Part of | Eyeball |
| System | Visual system |
| Function | Refract light |
| Identifiers | |
| Latin | lens crystallin |
| MeSH | D007908 |
| TA98 | A15.2.05.001 |
| TA2 | 6798 |
| FMA | 58241 |
| Anatomical terminology | |
The lens, or crystalline lens, is a transparent biconvex structure in most land vertebrate eyes. Along with the cornea, aqueous and vitreous humours it refracts light, focusing it onto the retina. In many land animals the shape of the lens can be altered, effectively changing the focal length of the eye, enabling them to focus on objects at various distances. This adjustment of the lens is known as accommodation (see also below). In many fully aquatic vertebrates such as fish other methods of accommodation are used such as changing the lens's position relative to the retina rather than changing lens shape. Accommodation is analogous to the focusing of a photographic camera via changing its lenses. In land vertebrates the lens is flatter on its anterior side than on its posterior side, while in fish the lens is often close to spherical.
Accommodation in humans is well studied and allows artificial means of supplementing our focus such as glasses for correction of sight as we age. The refractive power of a younger human lens in its natural environment is approximately 18 dioptres, roughly one-third of the eye's total power of about 60 dioptres. By 25 years of age the ability of the lens to alter the light path has reduced to 10 dioptres and accommodation continues to decline with age.
Structure
Position in the eye
The lens is located towards the front part of the vertebrate eye called the anterior segment which includes the cornea and iris positioned in front of the lens. The lens is held in place by the suspensory ligaments (Zonule of Zinn),[1] attaching the lens at its equator to the rest of the eye[2][3] through the ciliary body. Behind the lens is the jelly-like vitreous body which helps hold the lens in place. At the front of the lens is the liquid aqueous humor which bathes the lens with nutrients and other things. Land vertebrate lenses usually have an ellipsoid, biconvex shape. The front surface is less curved than the back. A human adult the lens is typically about 10mm in diameter and 4mm thick though changes shape with accommodation and size due to grow throughout a person's lifetime.[4]
Anatomy
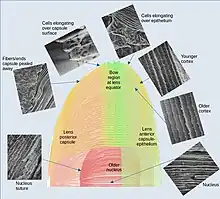


The lens has three main parts: the lens capsule, the lens epithelium, and the lens fibers. The lens capsule is a relatively thick basement membrane forming the outermost layer of the lens. Inside the capsule much thinner lens fibers form the bulk of the lens. The cells of the lens epithelium form a thin layer between the lens capsule and the outermost layer of lens fibers at the front of the lens but not the back. The lens itself lacks nerves, blood vessels, or connective tissue.[5] Anatomists will often refer to positions of structures in the lens by describing it like a globe of the world. The front and back of the lens are referred to as the anterior and posterior "poles", like the North and South poles. The "equator" is the outer edge of the lens often hidden by the iris and is the area of most cell differentiation. As the equator is not generally in the light path of the eye the structures involved with metabolic activity avoid scattering light that would otherwise affect vision.
Lens capsule
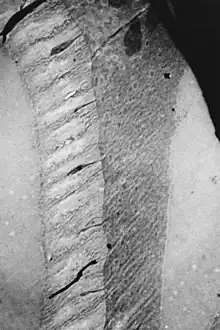
The lens capsule is a smooth, transparent basement membrane that completely surrounds the lens. The capsule is elastic and its main structural component is collagen. It is presumed to be synthesized by the lens epithelium and its main components in order of abundance are heparan sulfate proteoglycan (sulfated glycosaminoglycans (GAGs)), entactin, type IV collagen, laminin.[6] The capsule is very elastic and so allows the lens to assume a more spherical shape when the tension of the suspensory ligaments is reduced. The human capsule varies from 2 to 28 micrometres in thickness, being thickest near the equator (peri-equatorial region) and generally thinner near the posterior pole.[4] The photo from an electron microscope shows an area of the capsule near the equator where one of the thousands of suspensory ligaments attach.[7]
Attachment must be strong enough to stop the ligament being detached from the lens capsule. Forces are generated from holding the lens in place and added to when focusing. The anterior and posterior capsule is thinner.
Lens epithelium
The lens epithelium is a single layer of cells at the front of the lens between the lens capsule and the lens fibers.[4] By providing the lens fibers with nutrients and removing waste the cells of the epithelium regulate maintain lens homeostasis.[8] As ions, nutrients, and liquid enter the lens from the aqueous humor, Na+/K+-ATPase pumps in the lens epithelial cells pump ions out of the lens to maintain appropriate lens osmotic concentration and volume, with equatorially positioned lens epithelium cells contributing most to this current. The activity of the Na+/K+-ATPases keeps water and current flowing through the lens from the poles and exiting through the equatorial regions.
The cells of the lens epithelium also divide into new lens fibers at the lens equator.[9] The lens lays down fibers from when it first forms in embryo until death.[10]
Lens fibers
The lens fibers form the bulk of the lens. They are long, thin, transparent cells, firmly packed, with diameters typically 4–7 micrometres and lengths of up to 12mm long in humans.[4] The lens fibers stretch lengthwise from the posterior to the anterior poles and, when cut horizontally, are arranged in concentric layers rather like the layers of an onion. If cut along the equator, it appears as a honeycomb. The approximate middle of each fiber lies around the equator.[10] These tightly packed layers of lens fibers are referred to as laminae. The lens fiber cytoplasms are linked together via gap junctions, intercellular bridges and interdigitations of the cells that resemble "ball and socket" forms.
The lens is split into regions depending on the age of the lens fibers of a particular layer. Moving outwards from the central, oldest layer, the lens is split into an embryonic nucleus, the fetal nucleus, the adult nucleus, the inner and outer cortex. New lens fibers, generated from the lens epithelium, are added to the outer cortex. Mature lens fibers have no organelles or nuclei.
Cell fusion, voids and vacuoles
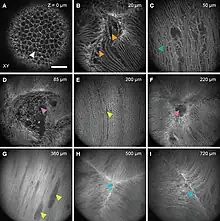

With the advent of other ways of looking at cellular structures of lenses while still in the living animal it became apparent that regions of fiber cells, at least at the lens anterior, contain large voids and vacuoles. These are speculated to be involved in lens transport systems linking the surface of the lens to deeper regions.[11] Very similar looking structures also indicate cell fusion in the lens. The cell fusion is shown by micro-injection to form a stratified syncytium in whole lens cultures.[9]
Development
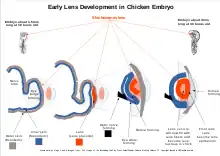
Development of the vertebrate lens begins when the human embryo is about 4mm long. The accompanying picture shows the process in a more easily studied chicken embryo. Unlike the rest of the eye which is derived mostly from the inner embryo layers, the lens is derived from the skin around the embryo. The first stage of lens formation takes place when a sphere of cells formed by budding of the inner embryo layers comes close to the embyro's outer skin. The sphere of cells induces nearby outer skin to start changing into the lens placode. The lens placode is the first stage of transformation of a patch of skin into the lens. At this early stage, the lens placode is a single layer of cells.[12][13]
As development progresses, the lens placode begins to deepen and bow inwards. As the placode continues to deepen, the opening to the surface ectoderm constricts[14] and the lens cells bud off from the embryo's skin to form a sphere of cells known as the "lens vesicle". When the embryo is about 10mm long the lens vesicle has completely separated from the skin of the embryo.
The embryo then sends signals from the developing retina, inducing the cells closest to the posterior end of the lens vesicle to elongate toward the anterior end of the vesicle.[14] These signals also induce the synthesis of proteins called crystallins.[15] As the name suggests the crystallins can form a clear highly refractive jelly. These elongating cells eventually fill in the center of the vesicle with cells, that are long and thin like a strand of hair, called fibers. These primary fibers become the nucleus in the mature lens. The epithelial cells that do not form into fibers nearest the lens front give rise to the lens epithelium.
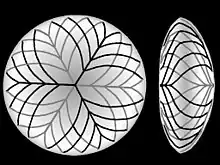
Additional fibers are derived from lens epithelial cells located at the lens equator. These cells lengthen towards the front and back wrapping around fibers already laid down. The new fibers need to be longer to cover earlier fibers but as the lens gets larger the ends of the newer fibers no longer reach as far towards the front and back of the lens. The lens fibers that do not reach the poles form tight, interdigitating seams with neighboring fibers. These seams being less crystalline then the bulk of the lens are more visible and are termed "sutures". The suture patterns become more complex as more layers of lens fibers are added to the outer portion of the lens.
The lens continues to grow after birth, with the new secondary fibers being added as outer layers. New lens fibers are generated from the equatorial cells of the lens epithelium, in a region referred to as the "germinative zone" and "bow region". The lens epithelial cells elongate, lose contact with the capsule and epithelium at the back and front of the lens, synthesize crystallin, and then finally lose their nuclei (enucleate) as they become mature lens fibers. In humans, as the lens grows by laying down more fibers through to early adulthood, the lens becomes more ellipsoid in shape. After about age 20 the lens grows rounder again and the iris is very important for this development.[4]
Several proteins control the embryonic development of the lens though PAX6 is considered the master regulator gene of this organ.[16] Other effectors of proper lens development include the Wnt signaling components BCL9 and Pygo2.[17] The whole process of differentiation of the epithelial cells into crystallin filled fiber cells without organelles occurs within the confines of the lens capsule. Older cells cannot be shed and are instead internalized towards the center of the lens. This process results in a complete temporally layered record of the differentiation process from the start at the lens surface to the end at the lens center. The lens is therefore valuable to scientists studying the process of cell differentiation.[18]
Variations in lens structure

In many aquatic vertebrates, the lens is considerably thicker, almost spherical resulting in increased light refraction. This difference helps compensate for the smaller angle of refraction between the eye's cornea and the watery environment, as they have more similar refractive indices than cornea and air.[19] The fiber cells of fish are generally considerably thinner than those of land vertebrates and it appears crystalin proteins are transported to the organelle free cells at the lens exterior to the inner cells through many layers of cells.[20] Some vertebrates need to see well both above and below water at times. One example is diving birds which have the ability to change focus by 50 to 80 dioptres. Compared with animals adapted for only one environment diving birds have a somewhat altered lens and cornea structure with focus mechanisms to allow for both environments.[21][22] Even among terrestrial animals the lens of primates such as humans is unusually flat going some way to explain why our vision, unlike diving birds, is particularly blurry under water.[23]
Function
Focusing

In humans the widely quoted Helmholtz mechanism of focusing, also called accommodation, is often referred to as a "model".[24] Direct experimental proof of any lens model is necessarily difficult as the vertebrate lens is transparent and only functions well in the living animals. When considering all vertebrates aspects of all models may play varying roles in lens focus.
The shape changing lens of many land based vertebrates
External forces

The model of a shape changing lens of humans was proposed by Young in a lecture on the 27th Nov 1800.[25] Others such as Helmholtz and Huxley refined the model in the mid 1800s explaining how the ciliary muscle contracts rounding the lens to focus near[26] and this model was popularized by Helmholtz in 1909.[27][28] The model may be summarized like this. Normally the lens is held under tension by its suspending ligaments being pulled tight by the pressure of the eyeball. At short focal distance the ciliary muscle contracts relieving some of the tension on the ligaments, allowing the lens to elastically round up a bit, increasing refractive power. Changing focus to an object at a greater distance requires a thinner less curved lens. This is achieved by relaxing some of the sphincter like ciliary muscles. While not referenced this presumably allows the pressure in the eyeball to again expand it outwards, pulling harder on the lens making it less curved and thinner, so increasing the focal distance. There is a problem with the Helmholtz model in that despite mathematical models being tried none has come close enough to working using only the Helmholtz mechanisms.[29]
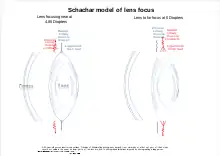
Schachar has proposed a model for land based vertebrates that was not well received.[30] The theory allows mathematical modeling to more accurately reflect the way the lens focuses while also taking into account the complexities in the suspensory ligaments and the presence of radial as well as circular muscles in the ciliary body.[31][32] In this model the ligaments may pull to varying degrees on the lens at the equator using the radial muscles while the ligaments offset from the equator to the front and back[33] are relaxed to varying degrees by contracting the circular muscles.[34] These multiple actions[35] operating on the elastic lens allows it to change lens shape at the front more subtly. Not only changing focus, but also correcting for lens aberrations that might otherwise result from the changing shape while better fitting mathematical modeling.[29]
The "catenary" model of lens focus proposed by Coleman[36] demands less tension on the ligaments suspending the lens. Rather than the lens as a whole being stretched thinner for distance vision and allowed to relax for near focus, contraction of the circular ciliary muscles results in the lens having less hydrostatic pressure against its front. The lens front can then reform its shape between the suspensory ligaments in a similar way to a slack chain hanging between two poles might change it's curve when the poles are moved closer together. This model requires fluid movement of the lens front only rather than trying to change the shape of the lens as a whole.
Internal forces

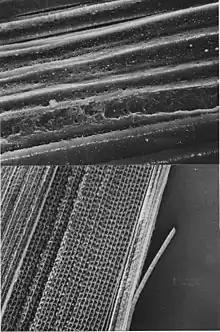
When Thomas Young proposed the changing of the human lens's shape as the mechanism for focal accommodation in 1801 he thought the lens may be a muscle capable of contraction. This type of model is termed intracapsular accommodation as it relies on activity within the lens. In a 1911 Nobel lecture Allvar Gullstrand spoke on "How I found the intracapsular mechanism of accommodation" and this aspect of lens focusing continues to be investigated.[37][38][39] Young spent time searching for the nerves that could stimulate the lens to contract without success. Since that time it has become clear the lens is not a simple muscle stimulated by a nerve so the 1909 Helmholtz model took precedence. Pre-twentieth century investigators did not have the benefit of many later discoveries and techniques. Membrane proteins such as aquaporins which allow water to flow into and out of cells are the most abundant membrane protein in the lens.[40][41] Connexins which allow electrical coupling of cells are also prevalent. Electron microscopy and immunofluorescent microscopy show fiber cells to be highly variable in structure and composition.[42][43][44] Magnetic resonance imaging confirms a layering in the lens that may allow for different refractive plans within it.[45] The refractive index of human lens varies from approximately 1.406 in the central layers down to 1.386 in less dense layers of the lens.[46] This index gradient enhances the optical power of the lens. As more is learned about mammalian lens structure from in situ Scheimpflug photography, MRI[47][48] and physiological investigations it is becoming apparent the lens itself is not responding entirely passively to the surrounding ciliary muscle but may be able to change its overall refractive index through mechanisms involving water dynamics in the lens still to be clarified.[49][50][51] The accompanying micrograph shows wrinkled fibers from a relaxed sheep lens after it is removed from the animal indicating shortening of the lens fibers during near focus accommodation. The age related changes in the human lens may also be related to changes in the water dynamics in the lens.[52][53]
Lenses of birds, reptiles, amphibians, fish and others
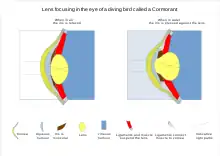

In reptiles and birds, the ciliary body which supports the lens via suspensory ligaments also touches the lens with a number of pads on its inner surface. These pads compress and release the lens to modify its shape while focusing on objects at different distances; the suspensory ligaments usually perform this function in mammals. With vision in fish and amphibians, the lens is fixed in shape, and focusing is instead achieved by moving the lens forwards or backwards within the eye using a muscle called the retractor lentus.[23]
In cartilaginous fish, the suspensory ligaments are replaced by a membrane, including a small muscle at the underside of the lens. This muscle pulls the lens forward from its relaxed position when focusing on nearby objects. In teleosts, by contrast, a muscle projects from a vascular structure in the floor of the eye, called the falciform process, and serves to pull the lens backwards from the relaxed position to focus on distant objects. While amphibians move the lens forward, as do cartilaginous fish, the muscles involved are not similar in either type of animal. In frogs, there are two muscles, one above and one below the lens, while other amphibians have only the lower muscle.[23]
In the simplest vertebrates, the lampreys and hagfish, the lens is not attached to the outer surface of the eyeball at all. There is no aqueous humor in these fish, and the vitreous body simply presses the lens against the surface of the cornea. To focus its eyes, a lamprey flattens the cornea using muscles outside of the eye and pushes the lens backwards.[23]
While not vertebrate, brief mention is made here of the convergent evolution of vertebrate and Molluscan eyes. The most complex Molluscan eye is the Cephalopod eye which is superficially similar structure and function to a vertebrate eye, including accommodation, while differing in basic ways such as having a two part lens and no cornea.[54][55] The fundamental requirements of optics must be filled by all eyes with lenses using the tissues at their disposal so superficially eyes all tend to look similar. It is the way optical requirements are met using different cell types and structural mechanisms that varies among animals.
Crystallins and transparency
Crystallins are water-soluble proteins that compose over 90% of the protein within the lens.[56] The three main crystallin types found in the human eye are α-, β-, and γ-crystallins. Crystallins tend to form soluble, high-molecular weight aggregates that pack tightly in lens fibers, thus increasing the index of refraction of the lens while maintaining its transparency. β and γ crystallins are found primarily in the lens, while subunits of α -crystallin have been isolated from other parts of the eye and the body. α-crystallin proteins belong to a larger superfamily of molecular chaperone proteins, and so it is believed that the crystallin proteins were evolutionarily recruited from chaperone proteins for optical purposes.[57] The chaperone functions of α-crystallin may also help maintain the lens proteins, which must last a human for their entire lifetime.[57]
Another important factor in maintaining the transparency of the lens is the absence of light-scattering organelles such as the nucleus, endoplasmic reticulum, and mitochondria within the mature lens fibers.[58] Lens fibers also have a very extensive cytoskeleton that maintains the precise shape and packing of the lens fibers; disruptions/mutations in certain cytoskeletal elements can lead to the loss of transparency.[59]
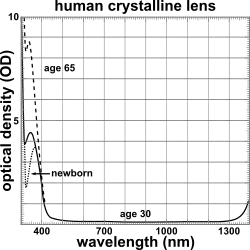
The lens blocks most ultraviolet light in the wavelength range of 300–400 nm; shorter wavelengths are blocked by the cornea. The pigment responsible for blocking the light is 3-hydroxykynurenine glucoside, a product of tryptophan catabolism in the lens epithelium.[60] High intensity ultraviolet light can harm the retina, and artificial intraocular lenses are therefore manufactured to also block ultraviolet light.[61] People lacking a lens (a condition known as aphakia) perceive ultraviolet light as whitish blue or whitish-violet.[62][63]
Nourishment
The lens is metabolically active and requires nourishment in order to maintain its growth and transparency. Compared to other tissues in the eye, however, the lens has considerably lower energy demands.[64]
By nine weeks into human development, the lens is surrounded and nourished by a net of vessels, the tunica vasculosa lentis, which is derived from the hyaloid artery.[15] Beginning in the fourth month of development, the hyaloid artery and its related vasculature begin to atrophy and completely disappear by birth.[65] In the postnatal eye, Cloquet's canal marks the former location of the hyaloid artery.
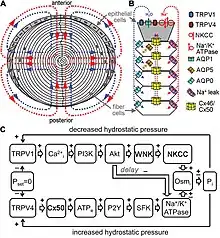
After regression of the hyaloid artery, the lens receives all its nourishment from the aqueous humor. Nutrients diffuse in and waste diffuses out through a constant flow of fluid from the anterior/posterior poles of the lens and out of the equatorial regions, a dynamic that is maintained by the Na+/K+-ATPase pumps located in the equatorially positioned cells of the lens epithelium.[8] The interaction of these pumps with water channels into cells called aquaporins, molecules less than 100 daltons in size among cells via gap junctions, and calcium using transporters/regulators (TRPV channels) results in a flow of nutrients throughout the lens.[66][67]
Glucose is the primary energy source for the lens. As mature lens fibers do not have mitochondria, approximately 80% of the glucose is metabolized via anaerobic metabolism.[68] The remaining fraction of glucose is shunted primarily down the pentose phosphate pathway.[68] The lack of aerobic respiration means that the lens consumes very little oxygen.[68]
Clinical significance
- Cataracts are opacities of the lens. While some are small and do not require any treatment, others may be large enough to block light and obstruct vision. Cataracts usually develop as the aging lens becomes more and more opaque, but cataracts can also form congenitally or after injury to the lens. Nuclear sclerosis is a type of age-related cataract. Diabetes is another risk factor for cataract. Cataract surgery involves the removal of the lens and insertion of an artificial intraocular lens.
- Presbyopia is the age-related loss of accommodation, which is marked by the inability of the eye to focus on nearby objects. The exact mechanism is still unknown, but age-related changes in the hardness, shape, and size of the lens have all been linked to the condition.
- Ectopia lentis is the displacement of the lens from its normal position.
- Aphakia is the absence of the lens from the eye. Aphakia can be the result of surgery or injury, or it can be congenital.
Additional images
 MRI scan of human eye showing lens.
MRI scan of human eye showing lens. Interior of anterior chamber of eye.
Interior of anterior chamber of eye.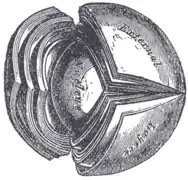 The crystalline lens, hardened and divided.
The crystalline lens, hardened and divided. Section through the margin of the lens, showing the transition of the epithelium into the lens fibers known as the bow region.
Section through the margin of the lens, showing the transition of the epithelium into the lens fibers known as the bow region.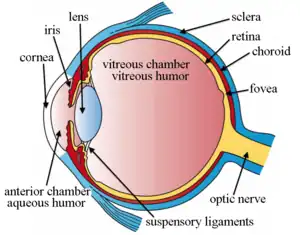 The structures of the eye labeled
The structures of the eye labeled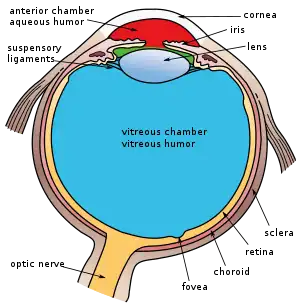 Another view of the eye and the structures of the eye labeled
Another view of the eye and the structures of the eye labeled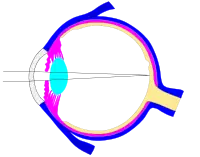 This svg file was configured so that the rays, diaphragm and crystalline lens are easily modified[69]
This svg file was configured so that the rays, diaphragm and crystalline lens are easily modified[69]
See also
- Accommodation reflex
- Crystallin
- Evolution of the eye, for how the lens evolved
- Intraocular lenses
- Iris
- Lens capsule
- Phacoemulsification
- Visual perception
- Zonules of Zinn
References
- ↑ Bassnett, Steven (May 2021). "Zinn's zonule". Progress in Retinal and Eye Research. 82: 100902. doi:10.1016/j.preteyeres.2020.100902. PMC 8139560. PMID 32980533.
- ↑ "Equator of lens - definition from". Biology-Online.org. Archived from the original on 2012-03-22. Retrieved 2012-11-25.
- ↑ "equator of the crystalline lens - definition of equator of the crystalline lens in the Medical dictionary - by the Free Online Medical Dictionary, Thesaurus and Encyclopedia". Medical-dictionary.thefreedictionary.com. Retrieved 2012-11-25.
- 1 2 3 4 5 John Forrester, Andrew Dick, Paul McMenamin, William Lee (1996). The Eye: Basic Sciences in Practice. London: W. B. Saunders Company Ltd. p. 28 ISBN 0-7020-1790-6
- ↑ Duker, Myron Yanoff, Jay S. (2008). Ophthalmology (3rd ed.). Edinburgh: Mosby. p. 382. ISBN 978-0323057516.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ↑ Mohan, PS; Spiro, RG (25 March 1986). "Macromolecular organization of basement membranes. Characterization and comparison of glomerular basement membrane and lens capsule components by immunochemical and lectin affinity procedures". The Journal of Biological Chemistry. 261 (9): 4328–36. doi:10.1016/S0021-9258(17)35665-X. PMID 3512568.
- ↑ Shi, Yanrong; Tu, Yidong; De Maria, Alicia; Mecham, Robert P.; Bassnett, Steven (1 April 2013). "Development, Composition, and Structural Arrangements of the Ciliary Zonule of the Mouse". Investigative Ophthalmology & Visual Science. 54 (4): 2504–2515. doi:10.1167/iovs.13-11619. PMC 3621578. PMID 23493297.
- 1 2 Candia, Oscar A. (2004). "Electrolyte and fluid transport across corneal, conjunctival and lens epithelia". Experimental Eye Research. 78 (3): 527–535. doi:10.1016/j.exer.2003.08.015. PMID 15106931.
- 1 2 Shi, Yanrong; Barton, Kelly; De Maria, Alicia; Petrash, J. Mark; Shiels, Alan; Bassnett, Steven (15 May 2009). "The stratified syncytium of the vertebrate lens". Journal of Cell Science. 122 (10): 1607–1615. doi:10.1242/jcs.045203. PMC 2680101. PMID 19401333.
- 1 2 "eye, human". Encyclopædia Britannica from Encyclopædia Britannica 2006 Ultimate Reference Suite DVD 2009
- ↑ Paidi, Santosh Kumar; Zhang, Qinrong; Yang, Yuhan; Xia, Chun-Hong; Ji, Na; Gong, Xiaohua (19 January 2023). "Adaptive optical two-photon fluorescence microscopy probes cellular organization of ocular lenses in vivo". bioRxiv 10.1101/2023.01.17.524320.
- ↑ Mitchell, PC (April 1891). "Double Chick Embryo". Journal of Anatomy and Physiology. 25 (Pt 3): 316–324.1. PMC 1328169. PMID 17231922.
- ↑ Chauhan, B; Plageman, T; Lou, M; Lang, R (2015). "Epithelial morphogenesis: the mouse eye as a model system". Current Topics in Developmental Biology. 111: 375–99. doi:10.1016/bs.ctdb.2014.11.011. PMC 6014593. PMID 25662266.
- 1 2 Muccioli, Maria; Qaisi, Dalya; Herman, Ken; Plageman, Timothy F. (April 2016). "Lens placode planar cell polarity is dependent on Cdc42-mediated junctional contraction inhibition". Developmental Biology. 412 (1): 32–43. doi:10.1016/j.ydbio.2016.02.016. PMC 7370377. PMID 26902112.
- 1 2 The Eye: Basic Sciences in Practice, p. 102, ISBN 0-7020-1790-6
- ↑ Cvekl, A.; Ashery-Padan, R. (2014). "The cellular and molecular mechanisms of vertebrate lens development". Development. 141 (23): 4432–4447. doi:10.1242/dev.107953. PMC 4302924. PMID 25406393.
- ↑ Cantù, Claudio; Zimmerli, Dario; Hausmann, George; Valenta, Tomas; Moor, Andreas; Aguet, Michel; Basler, Konrad (2014). "Pax6-dependent, but β-catenin-independent, function of Bcl9 proteins in mouse lens development". Genes & Development. 28 (17): 1879–1884. doi:10.1101/gad.246140.114. PMC 4197948. PMID 25184676.
- ↑ Limi, Saima; Senecal, Adrien; Coleman, Robert; Lopez-Jones, Melissa; Guo, Peng; Polumbo, Christina; Singer, Robert H.; Skoultchi, Arthur I.; Cvekl, Ales (August 2018). "Transcriptional burst fraction and size dynamics during lens fiber cell differentiation and detailed insights into the denucleation process". Journal of Biological Chemistry. 293 (34): 13176–13190. doi:10.1074/jbc.RA118.001927. PMC 6109918. PMID 29959226.
- ↑ Kardong, K. (2008). Vertebrates: Comparative anatomy, function, evolution (5th ed.). (pp. 676–677). Boston: McGraw-Hill
- ↑ Kozłowski, Tomasz M.; Kröger, Ronald H.H. (September 2019). "Constant lens fiber cell thickness in fish suggests crystallin transport to denucleated cells". Vision Research. 162: 29–34. doi:10.1016/j.visres.2019.06.008. PMID 31278970. S2CID 195820065.
- ↑ Katzir, Gadi; Howland, Howard C. (1 March 2003). "Corneal power and underwater accommodation in great cormorants( Phalacrocorax carbo sinensis )". Journal of Experimental Biology. 206 (5): 833–841. doi:10.1242/jeb.00142. PMID 12547938. S2CID 3096767.
- ↑ Sivak, J.G.; Hildebrand, T.; Lebert, C. (January 1985). "Magnitude and rate of accommodation in diving and nondiving birds". Vision Research. 25 (7): 925–933. doi:10.1016/0042-6989(85)90203-2. PMID 4049742. S2CID 42368520.
- 1 2 3 4 Romer, Alfred Sherwood; Parsons, Thomas S. (1977). The Vertebrate Body. Philadelphia, PA: Holt-Saunders International. pp. 463–464. ISBN 978-0-03-910284-5.
- ↑ Land, Michael (19 April 2015). "Focusing by shape change in the lens of the eye: a commentary on Young (1801) 'On the mechanism of the eye'". Philosophical Transactions of the Royal Society B: Biological Sciences. 370 (1666): 20140308. doi:10.1098/rstb.2014.0308. PMC 4360117. PMID 25750232.
- ↑ Land, M (19 April 2015). "Focusing by shape change in the lens of the eye: a commentary on Young (1801) 'On the mechanism of the eye'". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 370 (1666). doi:10.1098/rstb.2014.0308. PMC 4360117. PMID 25750232.
- ↑ Huxley, Thomas H (1871). Lessons in Elementary Physiology (5th ed.). London and New York: MACMILLAN AND CO. pp. 256–258.
- ↑ Helmholtz, H. von (1962). Treatise on physiological optics (English translation edited by JPC Southall. The Optical Society of America. From the third German Edition of Handbuch der Physiologischen Optik (1909), Leopold Voss, Leipzig. Dover reprint ed.). New York, NY: Dover Publications Inc.
- ↑ Fisher, R. F. (1 August 1977). "The force of contraction of the human ciliary muscle during accommodation". The Journal of Physiology. 270 (1): 51–74. doi:10.1113/jphysiol.1977.sp011938. PMC 1353417. PMID 915798.
- 1 2 Schachar, Ronald A.; Bax, Andrew J. (June 2001). "Mechanism of human accommodation as analyzed by nonlinear finite element analysis". Comprehensive Therapy. 27 (2): 122–132. doi:10.1007/s12019-996-0006-5. PMID 11430259. S2CID 71369369.
- ↑ Atchison, DA (July 1995). "Accommodation and presbyopia". Ophthalmic & Physiological Optics. 15 (4): 255–72. doi:10.1046/j.1475-1313.1995.9500020e.x. PMID 7667018. S2CID 24282106.
- ↑ Shao, Yilei; Tao, Aizhu; Jiang, Hong; Mao, Xinjie; Zhong, Jianguang; Shen, Meixiao; Lu, Fan; Xu, Zhe; Karp, Carol L.; Wang, Jianhua (1 June 2015). "Age-Related Changes in the Anterior Segment Biometry During Accommodation". Investigative Ophthalmology & Visual Science. 56 (6): 3522–3530. doi:10.1167/iovs.15-16825. PMC 4464043. PMID 26030106.
- ↑ Schachar, Ronald A. (22 September 2015). "Human Accommodative Ciliary Muscle Configuration Changes Are Consistent With Schachar's Mechanism of Accommodation". Investigative Ophthalmology & Visual Science. 56 (10): 6075. doi:10.1167/iovs.15-17452. PMID 26393665.
- ↑ Streeten, B W (1977). "B W Streeten; The zonular insertion: a scanning electron microscopic study". Invest. Ophthalmol. Vis. Sci. 16 (4): 364–375.
- ↑ Schachar, RA (March 1994). "Zonular function: a new hypothesis with clinical implications". Annals of Ophthalmology. 26 (2): 36–8. PMID 8010701.
- ↑ Knaus, Katherine R.; Hipsley, AnnMarie; Blemker, Silvia S. (June 2021). "The action of ciliary muscle contraction on accommodation of the lens explored with a 3D model". Biomechanics and Modeling in Mechanobiology. 20 (3): 879–894. doi:10.1007/s10237-021-01417-9. PMID 33491156. S2CID 231704221.
- ↑ Coleman, D. Jackson (June 1970). "Unified Model for Accommodative Mechanism". American Journal of Ophthalmology. 69 (6): 1063–1079. doi:10.1016/0002-9394(70)91057-3. PMID 5423772.
- ↑ PAU, H (1952). "[Accommodative shift of the nucleus of the lens in intracapsular accommodation]". Klinische Monatsblatter fur Augenheilkunde und fur augenarztliche Fortbildung. 121 (2): 224–6. PMID 14955961.
- ↑ Huggert, Arne (27 May 2009). "The Intracapsular Mechanism of Accommodation". Acta Ophthalmologica. 42 (2): 389–397. doi:10.1111/j.1755-3768.1964.tb03627.x. PMID 14213923. S2CID 37325357.
- ↑ López-Gil, Norberto (3 March 2022). "Gullstrand Intracapsular Accommodation Mechanism Revised". Photonics. 9 (3): 152. Bibcode:2022Photo...9..152L. doi:10.3390/photonics9030152.
- ↑ Broekhuyse, R. M.; Kuhlmann, E. D.; Stols, A. L. (September 1976). "Lens membranes II. Isolation and characterization of the main intrinsic polypeptide (MIP) of bovine lens fiber membranes". Experimental Eye Research. 23 (3): 365–371. doi:10.1016/0014-4835(76)90135-4. PMID 976377.
- ↑ Mulders, SM; Preston, GM; Deen, PM; Guggino, WB; van Os, CH; Agre, P (14 April 1995). "Water channel properties of major intrinsic protein of lens". The Journal of Biological Chemistry. 270 (15): 9010–16. doi:10.1074/jbc.270.15.9010. PMID 7536742.
- ↑ Kuszak, J; Alcala, J; Maisel, H (December 1980). "The surface morphology of embryonic and adult chick lens-fiber cells". The American Journal of Anatomy. 159 (4): 395–410. doi:10.1002/aja.1001590406. PMID 7223675.
- ↑ Gruijters, WT; Kistler, J; Bullivant, S (October 1987). "Formation, distribution and dissociation of intercellular junctions in the lens". Journal of Cell Science. 88 ( Pt 3) (3): 351–9. doi:10.1242/jcs.88.3.351. PMID 3448099.
- ↑ Gruijters, WT (July 1989). "A non-connexon protein (MIP) is involved in eye lens gap-junction formation". Journal of Cell Science. 93 ( Pt 3) (3): 509–13. doi:10.1242/jcs.93.3.509. PMID 2691517.
- ↑ Hermans, EA; Dubbelman, M; Van der Heijde, R; Heethaar, RM (December 2008). "Equivalent refractive index of the human lens upon accommodative response". Optometry and Vision Science. 85 (12): 1179–84. doi:10.1097/OPX.0b013e31818e8d57. PMID 19050472. S2CID 205907383.
- ↑ Hecht, Eugene. Optics, 2nd ed. (1987), Addison Wesley, ISBN 0-201-11609-X. p. 178.
- ↑ Hermans, Erik A.; Pouwels, Petra J. W.; Dubbelman, Michiel; Kuijer, Joost P. A.; van der Heijde, Rob G. L.; Heethaar, Rob M. (1 January 2009). "Constant Volume of the Human Lens and Decrease in Surface Area of the Capsular Bag during Accommodation: An MRI and Scheimpflug Study". Investigative Ophthalmology & Visual Science. 50 (1): 281–289. doi:10.1167/iovs.08-2124. PMID 18676625.
- ↑ Stahnke, T.; Hadlich, S.; Wree, A.; Guthoff, R.; Stachs, O.; Langner, S. (16 December 2016). "Magnetresonanzmikroskopie des Akkommodationsapparats". Klinische Monatsblätter für Augenheilkunde. 233 (12): 1320–1323. doi:10.1055/s-0042-118599. PMID 27984837. S2CID 78808282.
- ↑ Vaghefi, E; Pontre, BP; Jacobs, MD; Donaldson, PJ (August 2011). "Visualizing ocular lens fluid dynamics using MRI: manipulation of steady state water content and water fluxes". American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 301 (2): R335-42. doi:10.1152/ajpregu.00173.2011. PMID 21593426. S2CID 9525037.
- ↑ Donaldson, Paul J.; Chen, Yadi; Petrova, Rosica S.; Grey, Angus C.; Lim, Julie C. (December 2022). "Regulation of lens water content: Effects on the physiological optics of the lens". Progress in Retinal and Eye Research: 101152. doi:10.1016/j.preteyeres.2022.101152. PMID 36470825. S2CID 254243790.
- ↑ Suzuki-Kerr, H; Walker, KL; Han, MH; Lim, JC; Donaldson, PJ (2022). "Hyposmotic stress causes ATP release in a discrete zone within the outer cortex of rat lens". Molecular Vision. 28: 245–256. PMC 9514545. PMID 36284672.
- ↑ Moffat, BA; Landman, KA; Truscott, RJ; Sweeney, MH; Pope, JM (December 1999). "Age-related changes in the kinetics of water transport in normal human lenses". Experimental Eye Research. 69 (6): 663–9. doi:10.1006/exer.1999.0747. PMID 10620395.
- ↑ Jones, C.E.; Atchison, D.A.; Meder, R.; Pope, J.M. (August 2005). "Refractive index distribution and optical properties of the isolated human lens measured using magnetic resonance imaging (MRI)". Vision Research. 45 (18): 2352–2366. doi:10.1016/j.visres.2005.03.008. PMID 15979462. S2CID 8894700.
- ↑ Jagger, W. S; Sands, P. J (1 August 1999). "A wide-angle gradient index optical model of the crystalline lens and eye of the octopus". Vision Research. 39 (17): 2841–2852. doi:10.1016/S0042-6989(99)00012-7. PMID 10492814. S2CID 17808919.
- ↑ Schaeffel, F.; Murphy, C.J.; Howland, H.C. (15 November 1999). "Accommodation in the cuttlefish (Sepia officinalis)". Journal of Experimental Biology. 202 (22): 3127–3134. doi:10.1242/jeb.202.22.3127. PMID 10539961.
- ↑ Hoehenwarter, W.; Klose, J.; Jungblut, P. R. (2006). "Eye lens proteomics". Amino Acids. 30 (4): 369–389. doi:10.1007/s00726-005-0283-9. PMID 16583312. S2CID 19978371.
- 1 2 Andley, Usha P. (2007). "Crystallins in the eye: Function and pathology". Progress in Retinal and Eye Research. 26 (1): 78–98. doi:10.1016/j.preteyeres.2006.10.003. PMID 17166758. S2CID 29317220.
- ↑ Lang, Richard A. (January 1997). "Apoptosis in mammalian eye development: lens morphogenesis, vascular regression and immune privilege". Cell Death & Differentiation. 4 (1): 12–20. doi:10.1038/sj.cdd.4400211.
- ↑ Bloemendal, Hans; De Jong, Wilfried; Jaenicke, Rainer; Lubsen, Nicolette H.; Slingsby, Christine; Tardieu, Annette (2004). "Ageing and vision: Structure, stability and function of lens crystallins". Progress in Biophysics and Molecular Biology. 86 (3): 407–485. doi:10.1016/j.pbiomolbio.2003.11.012. PMID 15302206.
- ↑ Andrew M.Wood and Roger J.W.Truscott (March 1993). "UV Filters in Human Lenses: Tryptophan Catabolism". Experimental Eye Research. 56 (3): 317–325. doi:10.1006/exer.1993.1041. PMID 8472787.
- ↑ Mainster, M. A. (2006). "Violet and blue light blocking intraocular lenses: Photoprotection versus photoreception". British Journal of Ophthalmology. 90 (6): 784–792. doi:10.1136/bjo.2005.086553. PMC 1860240. PMID 16714268.
- ↑ Anderson, Robert M. (1983). "Visual Perceptions and Observations of an Aphakic Surgeon". Perceptual and Motor Skills. 57 (3_suppl): 1211–1218. doi:10.2466/pms.1983.57.3f.1211. PMID 6664798. S2CID 20005737.
- ↑ Hambling, David (29 May 2002). "Let the light shine in". The Guardian.
- ↑ Whikehart, David R. (2003). Biochemistry of the Eye, 2nd ed. 2003. Philadelphia: Butterworth Heinemann, p. 107–8 ISBN 0-7506-7152-1
- ↑ The Eye: Basic Sciences in Practice, p. 104, ISBN 0-7020-1790-6
- ↑ Giannone, Adrienne A.; Li, Leping; Sellitto, Caterina; White, Thomas W. (23 December 2021). "Physiological Mechanisms Regulating Lens Transport". Frontiers in Physiology. 12: 818649. doi:10.3389/fphys.2021.818649. PMC 8735835. PMID 35002784.
- ↑ Delamere, Nicholas A.; Shahidullah, Mohammad (31 January 2022). "Ion Transport Regulation by TRPV4 and TRPV1 in Lens and Ciliary Epithelium". Frontiers in Physiology. 12: 834916. doi:10.3389/fphys.2021.834916. PMC 8841554. PMID 35173627.
- 1 2 3 Biochemistry of the Eye, 2nd ed, p. 107–8, ISBN 0-7506-7152-1
- ↑ Download and open with Inkscape 9.1. The separate components reside on different "layers" to facilitated editing.
External links
- Histology image: 08001loa – Histology Learning System at Boston University
