Lodoxamide
 | |
| Names | |
|---|---|
IUPAC name
| |
| Clinical data | |
| Drug class | Mast cell stabilizer[1] |
| Main uses | Allergic conjunctivitis[2] |
| Side effects | Eye discomfort, dry eyes[1][2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Typical dose | 1 to 2 drops QID[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Chemical and physical data | |
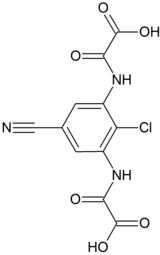
| Formula | C11H6ClN3O6 |
| Molar mass | 311.63 g·mol−1 |
Lodoxamide, sold under the brand name Alomide among others, is a medication used to treat allergic conjunctivitis.[2] It is used as an eye drop.[1] It may be used in children over the age of 2 years.[1]
Common side effects may include eye discomfort and dry eyes.[1][2] Use in pregnancy appears to be safe but such use has not been well studied.[3] It is a mast cell stabilizer.[1]
Lodoxamide was approved for medical use in the United States in 1993.[1] In the United Kingdom a 10 ml bottle costs the NHS about £5 as of 2021.[2] In the United States this amount costs about 175 USD.[4]
Research
In 2014 lodoxamide and bufrolin were found to be potent agonists at the G protein-coupled receptor 35, an orphan receptor believed to play a role in inflammatory processes, pain and the development of stomach cancer.[5]
References
- 1 2 3 4 5 6 7 8 "Lodoxamide Monograph for Professionals". Drugs.com. Archived from the original on 23 January 2021. Retrieved 24 November 2021.
- 1 2 3 4 5 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 1206. ISBN 978-0857114105.
- ↑ "Lodoxamide ophthalmic (Alomide) Use During Pregnancy". Drugs.com. Archived from the original on 26 November 2020. Retrieved 24 November 2021.
- ↑ "Lodoxamide Prices, Coupons & Savings Tips - GoodRx". GoodRx. Retrieved 24 November 2021.
- ↑ MacKenzie, AE; Caltabiano, G; Kent, TC; Jenkins, L; McCallum, JE; Hudson, BD; Nicklin, SA; Fawcett, L; Markwick, R; Charlton, SJ; Milligan, G (2014). "The antiallergic mast cell stabilizers lodoxamide and bufrolin as the first high and equipotent agonists of human and rat GPR35". Molecular Pharmacology. 85 (1): 91–104. doi:10.1124/mol.113.089482. ISSN 0026-895X. PMC 3868900. PMID 24113750.
External links
| Identifiers: |
|---|