MEAI
 | |
| Clinical data | |
|---|---|
| Trade names | Pace |
| Other names | 5-MeO-AI; 2,3-Dihydro-5-methoxy-1H-Inden-2-amine |
| Routes of administration | Oral |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | high |
| Metabolism | acetyl-aminoindandane |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
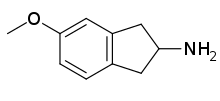
| Formula | C10H13NO |
| Molar mass | 163.220 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
MEAI (5-methoxy-2-aminoindane or 5-MeO-AI or Chaperon) belongs to the indane family of molecules. It was a recreational drug and binge drinking prevention drug. Its molecular structure was first mentioned implicitly in a markush structure schema appearing in a patent from 1998.[1] It was later explicitly and pharmacologically described in a peer reviewed paper in 2017 by David Nutt et al.,[2] followed by another in February 2018 which detailed pharmacokinetics, pharmacodynamics and metabolism of MEAI.[3] One year later it was studied an reported on in another peer reviewed paper.[4] In 2018, a company in the United States began offering an MEAI-based drink called "Pace".[5] The aminoindane family of molecules was, perhaps, first chemically described in 1980.[6][7]
MEAI was an early candidate of alcohol replacement drugs that came to market during a late 2010s movement to replace alcohol with less-toxic alternatives spearheaded by British psychopharmacologist David Nutt. MEAI was made commercially available online in the United States as "Pace" and is currently being prepared for FDA registration by Clearmind Medicine Inc (CSE:CMND).[8] The company claims wide Intellectual Property Holding[9][10][11]
See also
References
- ↑ US5708018A, Haadsma-Svensson, Susanne R.; Andersson, Bengt R. & Sonesson, Clas A. et al., "2-aminoindans as selective dopamine D3 ligands", issued 1998-01-13
- ↑ Shimshoni JA, Winkler I, Edery N, Golan E, van Wettum R, Nutt D (March 2017). "Toxicological evaluation of 5-methoxy-2-aminoindane (MEAI): Binge mitigating agent in development". Toxicology and Applied Pharmacology. 319: 59–68. doi:10.1016/j.taap.2017.01.018. PMID 28167221. S2CID 205304106.
- ↑ Shimshoni JA, Sobol E, Golan E, Ben Ari Y, Gal O (March 2018). "Pharmacokinetic and pharmacodynamic evaluation of 5-methoxy-2-aminoindane (MEAI): A new binge-mitigating agent". Toxicology and Applied Pharmacology. 343: 29–39. doi:10.1016/j.taap.2018.02.009. PMID 29458138. S2CID 3879333.
- ↑ Halberstadt, Adam L.; Brandt, Simon D.; Walther, Donna; Baumann, Michael H. (March 2019). "2-Aminoindan and its ring-substituted derivatives interact with plasma membrane monoamine transporters and α2-adrenergic receptors". Psychopharmacology. 236 (3): 989–999. doi:10.1007/s00213-019-05207-1. PMC 6848746.
- ↑ Pace D. "Pace by DACOA". Pace. DACOA. Retrieved 12 August 2018.
- ↑ Sainsbury PD, Kicman AT, Archer RP, King LA, Braithwaite RA (2011). "Aminoindanes--the next wave of 'legal highs'?". Drug Testing and Analysis. 3 (7–8): 479–82. doi:10.1002/dta.318. PMID 21748859.
- ↑ Cannon JG, Perez JA, Pease JP, Long JP, Flynn JR, Rusterholz DB, Dryer SE (July 1980). "Comparison of biological effects of N-alkylated congeners of beta-phenethylamine derived from 2-aminotetralin, 2-aminoindan, and 6-aminobenzocycloheptene". Journal of Medicinal Chemistry. 23 (7): 745–9. doi:10.1021/jm00181a009. PMID 7190613.
- ↑ "(CSE:CMND)"
- ↑ Alcoholic beverage substitutes
- ↑ European Patent - Binge Behavior Regulators
- ↑ Clearmind Medicine Inc.