Resmetirom
 | |
| Clinical data | |
|---|---|
| Other names | MGL-3196 |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
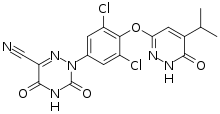
| Formula | C17H12Cl2N6O4 |
| Molar mass | 435.22 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Resmetirom is an experimental drug for the treatment of non-alcoholic steatohepatitis (NASH).[1][2] It is a selective agonist of thyroid hormone receptor-β which increases hepatic fat metabolism and reduces lipotoxicity.[1]
As of 2023, it is in Phase III clinical trials.[3][4]
References
- 1 2 Harrison SA, Bashir MR, Guy CD, Zhou R, Moylan CA, Frias JP, et al. (November 2019). "Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial". Lancet. 394 (10213): 2012–2024. doi:10.1016/S0140-6736(19)32517-6. PMID 31727409. S2CID 207976212.
- ↑ "Non-Alcoholic Fatty Liver Disease (NAFLD) Marketed and Pipeline Drugs Assessment, Clinical Trials and Competitive Landscape". Yahoo! Finance. January 23, 2023.
- ↑ Harrison SA. "A 52-Week Phase 3 Clinical Trial of Resmetirom in 180 Patients with Well-Compensated NASH Cirrhosis". AASLD: The Liver Meeting.
- ↑ "Madrigal Announces Positive Topline Results from the Pivotal Phase 3 MAESTRO-NASH Clinical Trial of Resmetirom for the Treatment of NASH and Liver Fibrosis" (Press release). Madrigal Pharmaceuticals. December 19, 2022.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.