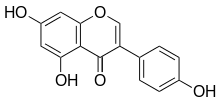
Genistein
 | |
 | |
| Names | |
|---|---|
| IUPAC name
4′,5,7-Trihydroxyisoflavone | |
| Preferred IUPAC name
5,7-Dihydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
Beilstein Reference |
263823 |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.006.524 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C15H10O5 |
| Molar mass | 270.240 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Genistein (C15H10O5) is a naturally occurring compound that structurally belongs to a class of compounds known as isoflavones. It is described as an angiogenesis inhibitor and a phytoestrogen.[1]
It was first isolated in 1899 from the dyer's broom, Genista tinctoria; hence, the chemical name. The compound structure was established in 1926, when it was found to be identical with that of prunetol. It was chemically synthesized in 1928.[2] It has been shown to be the primary secondary metabolite of the Trifolium species and Glycine max L.[3]
Natural occurrences
Isoflavones such as genistein and daidzein are found in a number of plants including lupin, fava beans, soybeans, kudzu, and psoralea being the primary food source,[4][5] also in the medicinal plants, Flemingia vestita[6] and F. macrophylla,[7][8] and coffee.[9] It can also be found in Maackia amurensis cell cultures.[10]
Biological effects
Besides functioning as an antioxidant and anthelmintic, many isoflavones have been shown to interact with animal and human estrogen receptors, causing effects in the body similar to those caused by the hormone estrogen. Isoflavones also produce non-hormonal effects.
Molecular function
Genistein influences multiple biochemical functions in living cells:
- full agonist of ERβ (EC50 = 7.62 nM) and, to a much lesser extent (~20-fold), full agonist[11] or partial agonist of ERα[12]
- agonist of G protein-coupled estrogen receptor (affinity of 133 nM)[13][14]
- activation of peroxisome proliferator-activated receptors (PPARs)
- inhibition of several tyrosine kinases
- inhibition of topoisomerase
- inhibition of AAAD
- direct antioxidation with some pro oxidative features
- activation of Nrf2 antioxidative response
- stimulation of autophagy[15][16][17]
- inhibition of the mammalian hexose transporter GLUT1
- contraction of several types of smooth muscles
- modulation of CFTR channel, potentiating its opening at low concentration and inhibiting it a higher doses.
- inhibition of cytosine methylation
- inhibition of DNA methyltransferase[18]
- inhibition of the glycine receptor
- inhibition of the nicotinic acetylcholine receptor[19]
Activation of PPARs
Isoflavones genistein and daidzein bind to and transactivate all three PPAR isoforms, α, δ, and γ.[20] For example, membrane-bound PPARγ-binding assay showed that genistein can directly interact with the PPARγ ligand binding domain and has a measurable Ki of 5.7 mM.[21] Gene reporter assays showed that genistein at concentrations between 1 and 100 uM activated PPARs in a dose dependent way in KS483 mesenchymal progenitor cells, breast cancer MCF-7 cells, T47D cells and MDA-MD-231 cells, murine macrophage-like RAW 264.7 cells, endothelial cells and in Hela cells. Several studies have shown that both ERs and PPARs influenced each other and therefore induce differential effects in a dose-dependent way. The final biological effects of genistein are determined by the balance among these pleiotrophic actions.[20][22][23]
Tyrosine kinase inhibitor
The main known activity of genistein is tyrosine kinase inhibitor, mostly of epidermal growth factor receptor (EGFR). Tyrosine kinases are less widespread than their ser/thr counterparts but implicated in almost all cell growth and proliferation signal cascades.
Redox-active—not only antioxidant
Genistein may act as direct antioxidant, similar to many other isoflavones, and thus may alleviate damaging effects of free radicals in tissues.[24][25]
The same molecule of genistein, similar to many other isoflavones, by generation of free radicals poison topoisomerase II, an enzyme important for maintaining DNA stability.[26][27][28]
Human cells turn on beneficial, detoxifying Nrf2 factor in response to genistein insult. This pathway may be responsible for observed health maintaining properties of small doses of genistein.[29]
Anthelmintic
The root-tuber peel extract of the leguminous plant Felmingia vestita is the traditional anthelmintic of the Khasi tribes of India. While investigating its anthelmintic activity, genistein was found to be the major isoflavone responsible for the deworming property.[6][30] Genistein was subsequently demonstrated to be highly effective against intestinal parasites such as the poultry cestode Raillietina echinobothrida,[30] the pork trematode Fasciolopsis buski,[31] and the sheep liver fluke Fasciola hepatica.[32] It exerts its anthelmintic activity by inhibiting the enzymes of glycolysis and glycogenolysis,[33][34] and disturbing the Ca2+ homeostasis and NO activity in the parasites.[35][36] It has also been investigated in human tapeworms such as Echinococcus multilocularis and E. granulosus metacestodes that genistein and its derivatives, Rm6423 and Rm6426, are potent cestocides.[37]
Atherosclerosis
Genistein protects against pro-inflammatory factor-induced vascular endothelial barrier dysfunction and inhibits leukocyte-endothelium interaction, thereby modulating vascular inflammation, a major event in the pathogenesis of atherosclerosis.[38]
Cancer links
Genistein and other isoflavones have been identified as angiogenesis inhibitors, and found to inhibit the uncontrolled cell growth of cancer, most likely by inhibiting the activity of substances in the body that regulate cell division and cell survival (growth factors). Various studies have found that moderate doses of genistein have inhibitory effects on cancers of the prostate,[39][40] cervix,[41] brain,[42] breast[39][43][44] and colon.[17] It has also been shown that genistein makes some cells more sensitive to radio-therapy.;[45] although, timing of phytoestrogen use is also important.[45]
Genistein's chief method of activity is as a tyrosine kinase inhibitor. Tyrosine kinases are less widespread than their ser/thr counterparts but implicated in almost all cell growth and proliferation signal cascades. Inhibition of DNA topoisomerase II also plays an important role in the cytotoxic activity of genistein.[27][46] The observation that transition of normal lymphocytes from quiescence (G0) to the G1 phase of the cell cycle is particularly sensitive to genistein prompted the authors to suggest that this isoflavone may be potential immunosuppressant.[47] Genistein has been used to selectively target pre B-cells via conjugation with an anti-CD19 antibody.[48]
Studies on rodents have found genistein to be useful in the treatment of leukemia, and that it can be used in combination with certain other antileukemic drugs to improve their efficacy.[49]
Estrogen receptor — more cancer links
Due to its structure similarity to 17β-estradiol (estrogen), genistein can compete with it and bind to estrogen receptors. However, genistein shows much higher affinity toward estrogen receptor β than toward estrogen receptor α.[50]
Data from in vitro and in vivo research confirms that genistein can increase rate of growth of some ER expressing breast cancers. Genistein was found to increase the rate of proliferation of estrogen-dependent breast cancer when not cotreated with an estrogen antagonist.[51][52][53] It was also found to decrease efficiency of tamoxifen and letrozole - drugs commonly used in breast cancer therapy.[54][55] Genistein was found to inhibit immune response towards cancer cells allowing their survival.[56]
Effects in males
Isoflavones can act like estrogen, stimulating development and maintenance of female characteristics, or they can block cells from using cousins of estrogen. In vitro studies have shown genistein to induce apoptosis of testicular cells at certain levels, thus raising concerns about effects it could have on male fertility;[57] however, one study found that isoflavones had "no observable effect on endocrine measurements, testicular volume or semen parameters over the study period." in healthy males given isoflavone supplements daily over a 2-month period.[58]
Carcinogenic and toxic potential
Genistein was, among other flavonoids, found to be a strong topoisomerase inhibitor, similarly to some chemotherapeutic anticancer drugs ex. etoposide and doxorubicin.[26][59] In high doses it was found to be strongly toxic to normal cells.[60] This effect may be responsible for both anticarcinogenic and carcinogenic potential of the substance.[28][61] It was found to deteriorate DNA of cultured blood stem cells, which may lead to leukemia.[62] Genistein among other flavonoids is suspected to increase risk of infant leukemia when consumed during pregnancy.[63][64]
Sanfilippo syndrome treatment
Genistein decreases pathological accumulation of glycosaminoglycans in Sanfilippo syndrome. In vitro animal studies and clinical experiments suggest that the symptoms of the disease may be alleviated by adequate dose of genistein.[65] Genistein was found to also possess toxic properties toward brain cells.[60] Among many pathways stimulated by genistein, autophagy may explain the observed efficiency of the substance as autophagy is significantly impaired in the disease.[66][67]
Related compounds
- Genistin is the 7-O-beta-D-glucoside of genistein.
- Wighteone can be described as 6-isopentenyl genistein
- KBU2046 under investigation for prostate cancer.[68][69]
- B43-genistein, an anti-CD19 antibody linked to genistein e.g. for leukemia.[70]
See also
References
- ↑ Sail, Vibhavari; Hadden, M. Kyle (2012-01-01), Desai, Manoj C. (ed.), Chapter Eighteen - Notch Pathway Modulators as Anticancer Chemotherapeutics, Annual Reports in Medicinal Chemistry, vol. 47, Academic Press, pp. 267–280, retrieved 2020-09-14
- ↑ Walter, E. D. (1941). "Genistin (an Isoflavone Glucoside) and its Aglucone, Genistein, from Soybeans". Journal of the American Chemical Society. 63 (12): 3273–76. doi:10.1021/ja01857a013.
- ↑ Popiołkiewicz, Joanna; Polkowski, Krzysztof; Skierski, Janusz S.; Mazurek, Aleksander P. (November 2005). "In vitro toxicity evaluation in the development of new anticancer drugs—genistein glycosides". Cancer Letters. 229 (1): 67–75. doi:10.1016/j.canlet.2005.01.014. ISSN 0304-3835. PMID 16157220.
- ↑ Coward, Lori; Barnes, Neil C.; Setchell, Kenneth D. R.; Barnes, Stephen (1993). "Genistein, daidzein, and their β-glycoside conjugates: Antitumor isoflavones in soybean foods from American and Asian diets". Journal of Agricultural and Food Chemistry. 41 (11): 1961–7. doi:10.1021/jf00035a027.
- ↑ Kaufman, Peter B.; Duke, James A.; Brielmann, Harry; Boik, John; Hoyt, James E. (1997). "A Comparative Survey of Leguminous Plants as Sources of the Isoflavones, Genistein and Daidzein: Implications for Human Nutrition and Health". The Journal of Alternative and Complementary Medicine. 3 (1): 7–12. CiteSeerX 10.1.1.320.9747. doi:10.1089/acm.1997.3.7. PMID 9395689.
- 1 2 Rao, H. S. P.; Reddy, K. S. (1991). "Isoflavones from Flemingia vestita". Fitoterapia. 62 (5): 458.
- ↑ Rao, K.Nageswara; Srimannarayana, G. (1983). "Fleminone, a flavanone from the stems of Flemingia macrophylla". Phytochemistry. 22 (10): 2287–90. doi:10.1016/S0031-9422(00)80163-6.
- ↑ Wang, Bor-Sen; Juang, Lih-Jeng; Yang, Jeng-Jer; Chen, Li-Ying; Tai, Huo-Mu; Huang, Ming-Hsing (2012). "Antioxidant and Antityrosinase Activity of Flemingia macrophylla and Glycine tomentella Roots". Evidence-Based Complementary and Alternative Medicine. 2012: 1–7. doi:10.1155/2012/431081. PMC 3444970. PMID 22997529.
- ↑ Alves, Rita C.; Almeida, Ivone M. C.; Casal, Susana; Oliveira, M. Beatriz P. P. (2010). "Isoflavones in Coffee: Influence of Species, Roast Degree, and Brewing Method". Journal of Agricultural and Food Chemistry. 58 (5): 3002–7. doi:10.1021/jf9039205. PMID 20131840.
- ↑ Fedoreyev, S.A; Pokushalova, T.V; Veselova, M.V; Glebko, L.I; Kulesh, N.I; Muzarok, T.I; Seletskaya, L.D; Bulgakov, V.P; Zhuravlev, Yu.N (2000). "Isoflavonoid production by callus cultures of Maackia amurensis". Fitoterapia. 71 (4): 365–72. doi:10.1016/S0367-326X(00)00129-5. PMID 10925005.
- ↑ Patisaul, Heather B.; Melby, Melissa; Whitten, Patricia L.; Young, Larry J. (2002). "Genistein Affects ERβ- But Not ERα-Dependent Gene Expression in the Hypothalamus". Endocrinology. 143 (6): 2189–2197. doi:10.1210/endo.143.6.8843. ISSN 0013-7227. PMID 12021182.
- ↑ Green, Sarah E (2015), In Vitro Comparison of Estrogenic Activities of Popular Women's Health Botanicals
- ↑ Prossnitz ER, Arterburn JB (July 2015). "International Union of Basic and Clinical Pharmacology. XCVII. G Protein-Coupled Estrogen Receptor and Its Pharmacologic Modulators". Pharmacol. Rev. 67 (3): 505–40. doi:10.1124/pr.114.009712. PMC 4485017. PMID 26023144.
- ↑ Prossnitz, Eric R.; Barton, Matthias (2014). "Estrogen biology: New insights into GPER function and clinical opportunities". Molecular and Cellular Endocrinology. 389 (1–2): 71–83. doi:10.1016/j.mce.2014.02.002. ISSN 0303-7207. PMC 4040308. PMID 24530924.
- ↑ Gossner, G; Choi, M; Tan, L; Fogoros, S; Griffith, K; Kuenker, M; Liu, J (2007). "Genistein-induced apoptosis and autophagocytosis in ovarian cancer cells". Gynecologic Oncology. 105 (1): 23–30. doi:10.1016/j.ygyno.2006.11.009. PMID 17234261.
- ↑ Singletary, K.; Milner, J. (2008). "Diet, Autophagy, and Cancer: A Review". Cancer Epidemiology, Biomarkers & Prevention. 17 (7): 1596–610. doi:10.1158/1055-9965.EPI-07-2917. PMID 18628411.
- 1 2 Nakamura, Yoshitaka; Yogosawa, Shingo; Izutani, Yasuyuki; Watanabe, Hirotsuna; Otsuji, Eigo; Sakai, Tosiyuki (2009). "A combination of indol-3-carbinol and genistein synergistically induces apoptosis in human colon cancer HT-29 cells by inhibiting Akt phosphorylation and progression of autophagy". Molecular Cancer. 8: 100. doi:10.1186/1476-4598-8-100. PMC 2784428. PMID 19909554.
- ↑ Fang, Mingzhu; Chen, Dapeng; Yang, Chung S. (January 2007). "Dietary polyphenols may affect DNA methylation". The Journal of Nutrition. 137 (1 Suppl): 223S–228S. doi:10.1093/jn/137.1.223S. PMID 17182830.
- ↑ Glushakov, A. V.; Glushakova, H. Y.; Skok, V. I. (1999-01-15). "Modulation of nicotinic acetylcholine receptor activity in submucous neurons by intracellular messengers". Journal of the Autonomic Nervous System. 75 (1): 16–22. doi:10.1016/S0165-1838(98)00165-9. ISSN 0165-1838. PMID 9935265.
- 1 2 Wang, Limei; Waltenberger, Birgit; Pferschy-Wenzig, Eva-Maria; Blunder, Martina; Liu, Xin; Malainer, Clemens; Blazevic, Tina; Schwaiger, Stefan; Rollinger, Judith M.; Heiss, Elke H.; Schuster, Daniela; Kopp, Brigitte; Bauer, Rudolf; Stuppner, Hermann; Dirsch, Verena M.; Atanasov, Atanas G. (2014). "Natural product agonists of peroxisome proliferator-activated receptor gamma (PPARγ): A review". Biochemical Pharmacology. 92 (1): 73–89. doi:10.1016/j.bcp.2014.07.018. PMC 4212005. PMID 25083916.
- ↑ Dang, Zhi-Chao; Audinot, Valérie; Papapoulos, Socrates E.; Boutin, Jean A.; Löwik, Clemens W. G. M. (2002). "Peroxisome Proliferator-activated Receptor γ (PPARγ) as a Molecular Target for the Soy Phytoestrogen Genistein". Journal of Biological Chemistry. 278 (2): 962–7. doi:10.1074/jbc.M209483200. PMID 12421816.
- ↑ Dang, Zhi Chao; Lowik, Clemens (2005). "Dose-dependent effects of phytoestrogens on bone". Trends in Endocrinology and Metabolism. 16 (5): 207–13. doi:10.1016/j.tem.2005.05.001. PMID 15922618. S2CID 35366615.
- ↑ Dang, Z. C. (2009). "Dose-dependent effects of soy phyto-oestrogen genistein on adipocytes: Mechanisms of action". Obesity Reviews. 10 (3): 342–9. doi:10.1111/j.1467-789X.2008.00554.x. PMID 19207876. S2CID 13804244.
- ↑ Han, Rui-Min; Tian, Yu-Xi; Liu, Yin; Chen, Chang-Hui; Ai, Xi-Cheng; Zhang, Jian-Ping; Skibsted, Leif H. (2009). "Comparison of Flavonoids and Isoflavonoids as Antioxidants". Journal of Agricultural and Food Chemistry. 57 (9): 3780–5. doi:10.1021/jf803850p. PMID 19296660.
- ↑ Borrás, Consuelo; Gambini, Juan; López-Grueso, Raúl; Pallardó, Federico V.; Viña, Jose (2010). "Direct antioxidant and protective effect of estradiol on isolated mitochondria" (PDF). Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 1802 (1): 205–11. doi:10.1016/j.bbadis.2009.09.007. PMID 19751829.
- 1 2 Bandele, Omari J.; Osheroff, Neil (2007). "Bioflavonoids as Poisons of Human Topoisomerase IIα and IIβ". Biochemistry. 46 (20): 6097–108. doi:10.1021/bi7000664. PMC 2893030. PMID 17458941.
- 1 2 Markovits, Judith; Linassier, Claude; Fossé, Philippe; Couprie, Jeanine; Pierre, Josiane; Jacquemin-Sablon, Alain; Saucier, Jean-Marie; Le Pecq, Jean-Bernard; Larsen, Annette K. (September 1989). "Inhibitory effects of the tyrosine kinase inhibitor genistein on mammalian DNA topoisomerase II". Cancer Research. 49 (18): 5111–7. PMID 2548712.
- 1 2 López-Lázaro, Miguel; Willmore, Elaine; Austin, Caroline A. (2007). "Cells Lacking DNA Topoisomerase IIβ are Resistant to Genistein". Journal of Natural Products. 70 (5): 763–7. doi:10.1021/np060609z. PMID 17411092.
- ↑ Mann, Giovanni E; Bonacasa, Barbara; Ishii, Tetsuro; Siow, Richard CM (2009). "Targeting the redox sensitive Nrf2–Keap1 defense pathway in cardiovascular disease: Protection afforded by dietary isoflavones". Current Opinion in Pharmacology. 9 (2): 139–45. doi:10.1016/j.coph.2008.12.012. PMID 19157984.
- 1 2 Tandon, V.; Pal, P.; Roy, B.; Rao, H. S. P.; Reddy, K. S. (1997). "In vitro anthelmintic activity of root-tuber extract of Flemingia vestita, an indigenous plant in Shillong, India". Parasitology Research. 83 (5): 492–8. doi:10.1007/s004360050286. PMID 9197399. S2CID 25086153.
- ↑ Kar, Pradip K; Tandon, Veena; Saha, Nirmalendu (2002). "Anthelmintic efficacy of Flemingia vestita: Genistein-induced effect on the activity of nitric oxide synthase and nitric oxide in the trematode parasite, Fasciolopsis buski". Parasitology International. 51 (3): 249–57. doi:10.1016/S1383-5769(02)00032-6. PMID 12243779.
- ↑ Toner, E.; Brennan, G. P.; Wells, K.; McGeown, J. G.; Fairweather, I. (2008). "Physiological and morphological effects of genistein against the liver fluke, Fasciola hepatica". Parasitology. 135 (10): 1189–203. doi:10.1017/S0031182008004630. PMID 18771609. S2CID 6525410.
- ↑ Tandon, Veena; Das, Bidyadhar; Saha, Nirmalendu (2003). "Anthelmintic efficacy of Flemingia vestita (Fabaceae): Effect of genistein on glycogen metabolism in the cestode, Raillietina echinobothrida". Parasitology International. 52 (2): 179–86. doi:10.1016/S1383-5769(03)00006-0. PMID 12798931.
- ↑ Das, B.; Tandon, V.; Saha, N. (2004). "Anthelmintic efficacy of Flemingia vestita (Fabaceae): Alteration in the activities of some glycolytic enzymes in the cestode, Raillietina echinobothrida". Parasitology Research. 93 (4): 253–61. doi:10.1007/s00436-004-1122-8. PMID 15138892. S2CID 9491127.
- ↑ Das, Bidyadhar; Tandon, Veena; Saha, Nirmalendu (2006). "Effect of isoflavone from Flemingia vestita (Fabaceae) on the Ca2+ homeostasis in Raillietina echinobothrida, the cestode of domestic fowl". Parasitology International. 55 (1): 17–21. doi:10.1016/j.parint.2005.08.002. PMID 16198617.
- ↑ Das, Bidyadhar; Tandon, Veena; Lyndem, Larisha M.; Gray, Alexander I.; Ferro, Valerie A. (2009). "Phytochemicals from Flemingia vestita (Fabaceae) and Stephania glabra (Menispermeaceae) alter cGMP concentration in the cestode Raillietina echinobothrida". Comparative Biochemistry and Physiology C. 149 (3): 397–403. doi:10.1016/j.cbpc.2008.09.012. PMID 18854226.
- ↑ Naguleswaran, Arunasalam; Spicher, Martin; Vonlaufen, Nathalie; Ortega-Mora, Luis M.; Torgerson, Paul; Gottstein, Bruno; Hemphill, Andrew (2006). "In Vitro Metacestodicidal Activities of Genistein and Other Isoflavones against Echinococcus multilocularis and Echinococcus granulosus". Antimicrobial Agents and Chemotherapy. 50 (11): 3770–8. doi:10.1128/AAC.00578-06. PMC 1635224. PMID 16954323.
- ↑ Si, Hongwei; Liu, Dongmin; Si, Hongwei; Liu, Dongmin (2007). "Phytochemical Genistein in the Regulation of Vascular Function: New Insights". Current Medicinal Chemistry. 14 (24): 2581–9. doi:10.2174/092986707782023325. PMID 17979711.
- 1 2 Morito, Keiko; Hirose, Toshiharu; Kinjo, Junei; Hirakawa, Tomoki; Okawa, Masafumi; Nohara, Toshihiro; Ogawa, Sumito; Inoue, Satoshi; Muramatsu, Masami; Masamune, Yukito (2001). "Interaction of Phytoestrogens with Estrogen Receptors α and β". Biological & Pharmaceutical Bulletin. 24 (4): 351–6. doi:10.1248/bpb.24.351. PMID 11305594.
- ↑ Hwang, Ye Won; Kim, Soo Young; Jee, Sun Ha; Kim, Youn Nam; Nam, Chung Mo (2009). "Soy Food Consumption and Risk of Prostate Cancer: A Meta-Analysis of Observational Studies". Nutrition and Cancer. 61 (5): 598–606. doi:10.1080/01635580902825639. PMID 19838933. S2CID 19719873.
- ↑ Kim, Su-Hyeon; Kim, Su-Hyeong; Kim, Yong-Beom; Jeon, Yong-Tark; Lee, Sang-Chul; Song, Yong-Sang (2009). "Genistein Inhibits Cell Growth by Modulating Various Mitogen-Activated Protein Kinases and AKT in Cervical Cancer Cells". Annals of the New York Academy of Sciences. 1171 (1): 495–500. Bibcode:2009NYASA1171..495K. doi:10.1111/j.1749-6632.2009.04899.x. PMID 19723095. S2CID 26111697.
- ↑ Das, Arabinda; Banik, Naren L.; Ray, Swapan K. (2009). "Flavonoids activated caspases for apoptosis in human glioblastoma T98G and U87MG cells but not in human normal astrocytes". Cancer. 116 (1): 164–76. doi:10.1002/cncr.24699. PMC 3159962. PMID 19894226.
- ↑ Sakamoto, Takako; Horiguchi, Hyogo; Oguma, Etsuko; Kayama, Fujio (2010). "Effects of diverse dietary phytoestrogens on cell growth, cell cycle and apoptosis in estrogen-receptor-positive breast cancer cells". The Journal of Nutritional Biochemistry. 21 (9): 856–64. doi:10.1016/j.jnutbio.2009.06.010. PMID 19800779.
- ↑ de Lemos, Mário L (2001). "Effects of Soy Phytoestrogens Genistein and Daidzein on Breast Cancer Growth". The Annals of Pharmacotherapy. 35 (9): 1118–21. doi:10.1345/aph.10257. PMID 11573864. S2CID 208876381.
- 1 2 de Assis, Sonia; Hilakivi-Clarke, Leena (2006). "Timing of Dietary Estrogenic Exposures and Breast Cancer Risk". Annals of the New York Academy of Sciences. 1089 (1): 14–35. Bibcode:2006NYASA1089...14D. doi:10.1196/annals.1386.039. PMID 17261753. S2CID 22170442.
- ↑ López-Lázaro, Miguel; Willmore, Elaine; Austin, Caroline A. (2007). "Cells Lacking DNA Topoisomerase IIβ are Resistant to Genistein". Journal of Natural Products. 70 (5): 763–7. doi:10.1021/np060609z. PMID 17411092.
- ↑ Traganos, F; Ardelt, B; Halko, N; Bruno, S; Darzynkiewicz, Z (1992). "Effects of genistein on the growth and cell cycle progression of normal human lymphocytes and human leukemic MOLT-4 and HL-60 cells". Cancer Res. 52 (22): 6200–8. PMID 1330289.
- ↑ Safa, Malek; Foon, Kenneth A.; Oldham, Robert K. (2009). "Drug Immunoconjugates". In Oldham, Robert K.; Dillman, Robert O. (eds.). Principles of Cancer Biotherapy (5th ed.). pp. 451–62. doi:10.1007/978-90-481-2289-9_12. ISBN 978-90-481-2277-6.
- ↑ Raynal, Noël J. M.; Charbonneau, Michel; Momparler, Louise F.; Momparler, Richard L. (2008). "Synergistic Effect of 5-Aza-2′-Deoxycytidine and Genistein in Combination Against Leukemia". Oncology Research Featuring Preclinical and Clinical Cancer Therapeutics. 17 (5): 223–30. doi:10.3727/096504008786111356. PMID 18980019.
- ↑ Kuiper, George G. J. M.; Lemmen, Josephine G.; Carlsson, Bo; Corton, J. Christopher; Safe, Stephen H.; van der Saag, Paul T.; van der Burg, Bart; Gustafsson, Jan-Åke (1998). "Interaction of Estrogenic Chemicals and Phytoestrogens with Estrogen Receptor β". Endocrinology. 139 (10): 4252–63. doi:10.1210/endo.139.10.6216. PMID 9751507.
- ↑ Ju, Young H.; Allred, Kimberly F.; Allred, Clinton D.; Helferich, William G. (2006). "Genistein stimulates growth of human breast cancer cells in a novel, postmenopausal animal model, with low plasma estradiol concentrations". Carcinogenesis. 27 (6): 1292–9. doi:10.1093/carcin/bgi370. PMID 16537557.
- ↑ Chen, Wen-Fang; Wong, Man-Sau (2004). "Genistein Enhances Insulin-Like Growth Factor Signaling Pathway in Human Breast Cancer (MCF-7) Cells". The Journal of Clinical Endocrinology & Metabolism. 89 (5): 2351–9. doi:10.1210/jc.2003-032065. PMID 15126563.
- ↑ Yang, Xiaohe; Yang, Shihe; McKimmey, Christine; Liu, Bolin; Edgerton, Susan M.; Bales, Wesley; Archer, Linda T.; Thor, Ann D. (2010). "Genistein induces enhanced growth promotion in ER-positive/erbB-2-overexpressing breast cancers by ER-erbB-2 cross talk and p27/kip1 downregulation". Carcinogenesis. 31 (4): 695–702. doi:10.1093/carcin/bgq007. PMID 20067990.
- ↑ Helferich, W. G.; Andrade, J. E.; Hoagland, M. S. (2008). "Phytoestrogens and breast cancer: A complex story". Inflammopharmacology. 16 (5): 219–26. doi:10.1007/s10787-008-8020-0. PMID 18815740. S2CID 11659490.
- ↑ Tonetti, Debra A.; Zhang, Yiyun; Zhao, Huiping; Lim, Sok-Bee; Constantinou, Andreas I. (2007). "The Effect of the Phytoestrogens Genistein, Daidzein, and Equol on the Growth of Tamoxifen-Resistant T47D/PKCα". Nutrition and Cancer. 58 (2): 222–9. doi:10.1080/01635580701328545. PMID 17640169. S2CID 10831895.
- ↑ Jiang, Xinguo; Patterson, Nicole M.; Ling, Yan; Xie, Jianwei; Helferich, William G.; Shapiro, David J. (2008). "Low Concentrations of the Soy Phytoestrogen Genistein Induce Proteinase Inhibitor 9 and Block Killing of Breast Cancer Cells by Immune Cells". Endocrinology. 149 (11): 5366–73. doi:10.1210/en.2008-0857. PMC 2584580. PMID 18669594.
- ↑ Kumi-Diaka, James; Rodriguez, Rosanna; Goudaze, Gould (1998). "Influence of genistein (4′,5,7-trihydroxyisoflavone) on the growth and proliferation of testicular cell lines". Biology of the Cell. 90 (4): 349–54. doi:10.1016/S0248-4900(98)80015-4. PMID 9800352.
- ↑ Mitchell, Julie H.; Cawood, Elizabeth; Kinniburgh, David; Provan, Anne; Collins, Andrew R.; Irvine, D. Stewart (2001). "Effect of a phytoestrogen food supplement on reproductive health in normal males". Clinical Science. 100 (6): 613–8. doi:10.1042/CS20000212. PMID 11352776.
- ↑ Lutz, Werner K.; Tiedge, Oliver; Lutz, Roman W.; Stopper, Helga (2005). "Different Types of Combination Effects for the Induction of Micronuclei in Mouse Lymphoma Cells by Binary Mixtures of the Genotoxic Agents MMS, MNU, and Genistein". Toxicological Sciences. 86 (2): 318–23. doi:10.1093/toxsci/kfi200. PMID 15901918.
- 1 2 Jin, Ying; Wu, Heng; Cohen, Eric M.; Wei, Jianning; Jin, Hong; Prentice, Howard; Wu, Jang-Yen (2007). "Genistein and daidzein induce neurotoxicity at high concentrations in primary rat neuronal cultures". Journal of Biomedical Science. 14 (2): 275–84. doi:10.1007/s11373-006-9142-2. PMID 17245525.
- ↑ Schmidt, Friederike; Knobbe, Christiane; Frank, Brigitte; Wolburg, Hartwig; Weller, Michael (2008). "The topoisomerase II inhibitor, genistein, induces G2/M arrest and apoptosis in human malignant glioma cell lines". Oncology Reports. 19 (4): 1061–6. doi:10.3892/or.19.4.1061. PMID 18357397.
- ↑ van Waalwijk van Doorn-Khosrovani, Sahar Barjesteh; Janssen, Jannie; Maas, Lou M.; Godschalk, Roger W. L.; Nijhuis, Jan G.; van Schooten, Frederik J. (2007). "Dietary flavonoids induce MLL translocations in primary human CD34+ cells". Carcinogenesis. 28 (8): 1703–9. doi:10.1093/carcin/bgm102. PMID 17468513.
- ↑ Spector, Logan G.; Xie, Yang; Robison, Leslie L.; Heerema, Nyla A.; Hilden, Joanne M.; Lange, Beverly; Felix, Carolyn A.; Davies, Stella M.; Slavin, Joanne; Potter, John D.; Blair, Cindy K.; Reaman, Gregory H.; Ross, Julie A. (2005). "Maternal Diet and Infant Leukemia: The DNA Topoisomerase II Inhibitor Hypothesis: A Report from the Children's Oncology Group". Cancer Epidemiology, Biomarkers & Prevention. 14 (3): 651–5. doi:10.1158/1055-9965.EPI-04-0602. PMID 15767345.
- ↑ Azarova, Anna M.; Lin, Ren-Kuo; Tsai, Yuan-Chin; Liu, Leroy F.; Lin, Chao-Po; Lyu, Yi Lisa (2010). "Genistein induces topoisomerase IIbeta- and proteasome-mediated DNA sequence rearrangements: Implications in infant leukemia". Biochemical and Biophysical Research Communications. 399 (1): 66–71. doi:10.1016/j.bbrc.2010.07.043. PMC 3376163. PMID 20638367.
- ↑ Piotrowska, Ewa; Jakóbkiewicz-Banecka, Joanna; Barańska, Sylwia; Tylki-Szymańska, Anna; Czartoryska, Barbara; Węgrzyn, Alicja; Węgrzyn, Grzegorz (2006). "Genistein-mediated inhibition of glycosaminoglycan synthesis as a basis for gene expression-targeted isoflavone therapy for mucopolysaccharidoses". European Journal of Human Genetics. 14 (7): 846–52. doi:10.1038/sj.ejhg.5201623. PMID 16670689.
- ↑ Ballabio, A. (2009). "Disease pathogenesis explained by basic science: Lysosomal storage diseases as autophagocytic disorders". International Journal of Clinical Pharmacology and Therapeutics. 47 (Suppl 1): S34–8. doi:10.5414/cpp47034. PMID 20040309.
- ↑ Settembre, Carmine; Fraldi, Alessandro; Jahreiss, Luca; Spampanato, Carmine; Venturi, Consuelo; Medina, Diego; de Pablo, Raquel; Tacchetti, Carlo; Rubinsztein, David C.; Ballabio, Andrea (2007). "A block of autophagy in lysosomal storage disorders". Human Molecular Genetics. 17 (1): 119–29. doi:10.1093/hmg/ddm289. PMID 17913701.
- ↑ Xu, Li; Farmer, Rebecca; Huang, Xiaoke; Pavese, Janet; Voll, Eric; Irene, Ogden; Biddle, Margaret; Nibbs, Antoinette; Valsecchi, Matias; Scheidt, Karl; Bergan, Raymond (2010). "Abstract B58: Discovery of a novel drug KBU2046 that inhibits conversion of human prostate cancer to a metastatic phenotype". Cancer Prevention Research. 3 (12 Supplement): B58. doi:10.1158/1940-6207.PREV-10-B58.
- ↑ "New Drug Stops Spread of Prostate Cancer" (Press release). Northwestern University. April 3, 2012. Retrieved September 27, 2014.
- ↑ Chen, Chun-Lin; Levine, Alexandra; Rao, Asha; O'Neill, Karen; Messinger, Yoav; Myers, Dorothea E.; Goldman, Frederick; Hurvitz, Carole; Casper, James T.; Uckun, Fatih M. (1999). "Clinical Pharmacokinetics of the CD19 Receptor-Directed Tyrosine Kinase Inhibitor B43-Genistein in Patients with B-Lineage Lymphoid Malignancies". The Journal of Clinical Pharmacology. 39 (12): 1248–55. doi:10.1177/00912709922012051. PMID 10586390. S2CID 24445516.
External links
| Wikimedia Commons has media related to Genistein. |