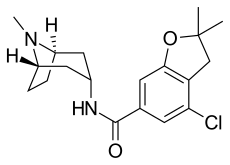
Zatosetron
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C19H25ClN2O2 |
| Molar mass | 348.87 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Zatosetron (LY-277,359) is a drug which acts as an antagonist at the 5HT3 receptor[1] It is orally active and has a long duration of action, producing antinauseant effects but without stimulating the rate of gastrointestinal transport.[2][3] It is also an effective anxiolytic in both animal studies and human trials,[4] although with some side effects at higher doses.[5][6]
See also
References
- ↑ Cohen ML, Bloomquist W, Gidda JS, Lacefield W (July 1990). "LY277359 maleate: a potent and selective 5-HT3 receptor antagonist without gastroprokinetic activity". The Journal of Pharmacology and Experimental Therapeutics. 254 (1): 350–5. PMID 2366187.
- ↑ Robertson DW, Lacefield WB, Bloomquist W, Pfeifer W, Simon RL, Cohen ML (January 1992). "Zatosetron, a potent, selective, and long-acting 5HT3 receptor antagonist: synthesis and structure-activity relationships". Journal of Medicinal Chemistry. 35 (2): 310–9. doi:10.1021/jm00080a016. PMID 1732548.
- ↑ Schwartz SM, Goldberg MJ, Gidda JS, Cerimele BJ (March 1994). "Effect of zatosetron on ipecac-induced emesis in dogs and healthy men". Journal of Clinical Pharmacology. 34 (3): 250–4. doi:10.1002/j.1552-4604.1994.tb03994.x. PMID 7517409. S2CID 23486859.
- ↑ Smith WT, Londborg PD, Blomgren SL, Tollefson GD, Sayler ME (April 1999). "Pilot study of zatosetron (LY277359) maleate, a 5-hydroxytryptamine-3 antagonist, in the treatment of anxiety". Journal of Clinical Psychopharmacology. 19 (2): 125–31. doi:10.1097/00004714-199904000-00006. PMID 10211913.
- ↑ Williams PD, Calligaro DO, Colbert WE, Helton DR, Shetler T, Turk JA, Jordan WH (March 1991). "General pharmacology of a new potent 5-hydroxytryptamine antagonist". Arzneimittel-Forschung. 41 (3): 189–95. PMID 1867653.
- ↑ Bendele A, Means J, Shoufler J, Schmalz C, Hanasono G, Symanowski J, Adams E (February 1995). "Chronic toxicity of zatosetron, a 5-HT3 receptor antagonist, in rhesus monkeys". Drug and Chemical Toxicology. 18 (1): 61–82. doi:10.3109/01480549509017858. PMID 7768200.
| 5-HT1AR agonists | |
|---|---|
| GABAAR PAMs |
|
| Gabapentinoids (α2δ VDCC blockers) | |
| Antidepressants |
|
| Sympatholytics (Antiadrenergics) |
|
| Others | |
| |
Glycine receptor modulators | |||||
|---|---|---|---|---|---|
| Receptor (ligands) |
| ||||
| Transporter (blockers) |
| ||||
| |||||
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.