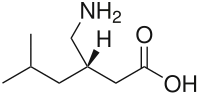
Pregabalin
 | |
 | |
| Names | |
|---|---|
| Pronunciation | /priˈɡæbəlɪn/ |
| Trade names | Lyrica, others[1] |
| Other names | 3-isobutyl GABA, (S)-3-isobutyl-γ-aminobutyric acid |
IUPAC name
| |
| Clinical data | |
| Drug class | Gabapentinoid |
| Main uses | Epilepsy, neuropathic pain, fibromyalgia, restless leg syndrome, generalized anxiety disorder[2][3][4] |
| Side effects | Headache, dizziness, sleepiness, confusion, trouble with memory, poor coordination, dry mouth, problem with vision, weight gain[2][5] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Dependence risk | Physical: Moderate[6] Psychological: Moderate[6] |
| Addiction risk | Low[6] |
| Pregnancy category | |
| Routes of use | By mouth |
| Onset of action | May occur within a week (pain)[8] |
| Duration of action | Unknown[9] |
| Defined daily dose | 300 mg[10] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a605045 |
| Legal | |
| License data |
|
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | High (≥90% rapidly absorbed; administration with food has no significant effect on bioavailability)[11] |
| Protein binding | <1%[12] |
| Metabolites | N-methylpregabalin[11] |
| Elimination half-life | 6.3–11.5 hours[13][14][15] |
| Excretion | Kidney |
| Chemical and physical data | |
| Formula | C8H17NO2 |
| Molar mass | 159.229 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Pregabalin, marketed under the brand name Lyrica among others, is a medication used to treat epilepsy, neuropathic pain, fibromyalgia, restless leg syndrome, and generalized anxiety disorder.[2][3][4] Its use in epilepsy is as an add-on therapy for partial seizures.[2] When used before surgery, it reduces pain but results in greater sedation and visual disturbances.[16] It is taken by mouth.[2]
Common side effects include headache, dizziness, sleepiness, confusion, trouble with memory, poor coordination, dry mouth, problem with vision, and weight gain.[2][5] Serious side effects may include angioedema, drug misuse, and an increased suicide risk.[2] When pregabalin is taken at high doses over a long period of time, addiction may occur, but if taken at usual doses the risk is low.[6] Use during the first trimester of pregnancy may slightly raise the risk of birth defects.[17] Use during breastfeeding is of unclear safety.[18] Pregabalin is a gabapentinoid and acts by inhibiting certain calcium channels.[19][20]
Pregabalin was approved for medical use in the United States in 2004.[2] It was developed as a successor to gabapentin.[21] It is available as a generic medication in a number of countries, including the United States as of 2019.[5][22][23] In the US the wholesale cost is about US$11 per month as of 2019.[24] While in the United Kingdom a similar dose costs the NHS about £6 as of 2018.[5] In 2017, it was the 72nd most prescribed medication in the United States with more than 11 million prescriptions.[25][26] In the US, Pregabalin is a Schedule V controlled substance.[2] It is a Class C controlled substance in the UK.[27] In France it is the most commonly forged prescription.[28]
Medical uses
Seizures
Pregabalin is useful when added to other treatments, when those other treatments are not controlling partial epilepsy.[29] Its use alone is less effective than some other seizure medications.[30] It is unclear how it compares to gabapentin for this use.[30]
Neuropathic pain
The European Federation of Neurological Societies recommends pregabalin as a first line agent for the treatment of pain associated with diabetic neuropathy, post-herpetic neuralgia, and central neuropathic pain.[31] A minority obtain substantial benefit, and a larger number obtain moderate benefit.[32] It is given equal weight as gabapentin and tricyclic antidepressants as a first line agent, however the latter are less expensive as of 2010.[33] Evidence does not support it being useful in sciatica or low back pain.[34]
Pregabalin's use in cancer-associated neuropathic pain is controversial;[35] though such use is common.[36] There is no evidence for its use in the prevention of migraines and gabapentin has also been found not to be useful.[37] It has been examined for the prevention of post-surgical chronic pain, but its utility for this purpose is controversial.[38][39]
Pregabalin is generally not regarded as efficacious in the treatment of acute pain.[32] In trials examining the utility of pregabalin for the treatment of acute post-surgical pain, no effect on overall pain levels was observed, but people did require less morphine and had fewer opioid-related side effects.[38][40] Several possible mechanisms for pain improvement have been discussed.[41]
Anxiety disorders
Pregabalin appears moderately effective for generalized anxiety disorder; though there are concerns about the quality of the evidence.[42][43] The World Federation of Biological Psychiatry recommends it as one of several first line agents for the treatment of generalized anxiety disorder, but recommends other agents such as SSRIs as first line treatment for obsessive–compulsive disorder and post-traumatic stress disorder.[44] It appears to have anxiolytic effects similar to benzodiazepines with less risk of dependence.[45][46]
The effects of pregabalin appear after 1 week of use and are similar in effectiveness to lorazepam, alprazolam, and venlafaxine, but pregabalin has demonstrated superiority by producing more consistent therapeutic effects for psychosomatic anxiety symptoms.[47] Long-term trials have shown continued effectiveness without the development of tolerance, and, in addition, unlike benzodiazepines, it has a beneficial effect on sleep and sleep architecture, characterized by the enhancement of slow-wave sleep.[47] It produces less severe cognitive and psychomotor impairment compared to those drugs; it also has a low potential for abuse and dependence and may be preferred over the benzodiazepines for these reasons.[47][48]
Other uses
Evidence finds little benefit and significant risk in those with chronic low back pain.[49][50] Evidence of benefit in alcohol withdrawal is poor as of 2016.[51]
Dosage
The defined daily dose is 300 mg by mouth.[10]
Side effects
Exposure to pregabalin is associated with weight gain, sleepiness and fatigue, dizziness, vertigo, leg swelling, disturbed vision, loss of coordination, and euphoria.[52] It has an adverse effect profile similar to other central nervous system depressants.[53] Adverse drug reactions associated with the use of pregabalin include:[54][55]
- Very common (>10% of people with pregabalin): dizziness, drowsiness.
- Common (1–10% of people with pregabalin): blurred vision, diplopia, increased appetite and subsequent weight gain, euphoria, confusion, vivid dreams, changes in libido (increase or decrease), irritability, ataxia, attention changes, feeling high, abnormal coordination, memory impairment, tremors, dysarthria, parasthesia, vertigo, dry mouth and constipation, vomiting and flatulence, erectile dysfunction, fatigue, peripheral edema, feeling the effects of drunkenness, abnormal walking, asthenia, nasopharyngitis, increased creatine kinase level.
- Infrequent (0.1–1% of people with pregabalin): depression, lethargy, agitation, anorgasmia, hallucinations, myoclonus, hypoaesthesia, hyperaesthesia, tachycardia, excessive salivation, hypoglycaemia, sweating, flushing, rash, muscle cramp, myalgia, arthralgia, urinary incontinence, dysuria, thrombocytopenia, kidney calculus
- Rare (<0.1% of people with pregabalin): neutropenia, first degree heart block, hypotension, hypertension, pancreatitis, dysphagia, oliguria, rhabdomyolysis, suicidal thoughts or behavior.[56]
Case of recreational use, with associated adverse effects has been reported.[57]
Withdrawal symptoms
Following abrupt or rapid discontinuation of pregabalin, some people reported symptoms suggestive of physical dependence. The FDA determined that the substance dependence profile of pregabalin, as measured by a personal physical withdrawal checklist, was quantitatively less than benzodiazepines.[53] Even people who have discontinued short term use of pregabalin have experienced withdrawal symptoms, including insomnia, headache, nausea, anxiety, diarrhea, flu like symptoms, nervousness, major depression, pain, convulsions, hyperhidrosis and dizziness.[58]
Pregnancy
It is unclear if it is safe for use in pregnancy with some studies showing potential harm.[59]
Breathing
In December 2019, the U.S. Food and Drug Administration (FDA) warned about serious breathing issues for those taking gabapentin or pregabalin when used with CNS depressants or for those with lung problems.[60][61]
The FDA required new warnings about the risk of respiratory depression to be added to the prescribing information of the gabapentinoids.[60] The FDA also required the drug manufacturers to conduct clinical trials to further evaluate their abuse potential, particularly in combination with opioids, because misuse and abuse of these products together is increasing, and co-use may increase the risk of respiratory depression.[60]
Among 49 case reports submitted to the FDA over the five-year period from 2012 to 2017, twelve people died from respiratory depression with gabapentinoids, all of whom had at least one risk factor.[60]
The FDA reviewed the results of two randomized, double-blind, placebo-controlled clinical trials in healthy people, three observational studies, and several studies in animals.[60] One trial showed that using pregabalin alone and using it with an opioid pain reliever can depress breathing function.[60] The other trial showed gabapentin alone increased pauses in breathing during sleep.[60] The three observational studies at one academic medical center showed a relationship between gabapentinoids given before surgery and respiratory depression occurring after different kinds of surgeries.[60] The FDA also reviewed several animal studies that showed pregabalin alone and pregabalin plus opioids can depress respiratory function.[60]
Overdose
Several people with kidney failure developed myoclonus while receiving pregabalin, apparently as a result of gradual accumulation of the drug. Acute overdosage may be manifested by somnolence, tachycardia and hypertonia. Plasma, serum or blood concentrations of pregabalin may be measured to monitor therapy or to confirm a diagnosis of poisoning in hospitalized people.[62][63][64]
Interactions
No interactions have been demonstrated in vivo. The manufacturer notes some potential pharmacological interactions with opioids, benzodiazepines, barbiturates, ethanol (alcohol), and other drugs that depress the central nervous system. ACE inhibitors may enhance the adverse/toxic effect of pregabalin. Pregabalin may enhance the fluid-retaining effect of anti-diabetic agents (thiazolidinedione).[65]
Pharmacology
Pharmacodynamics
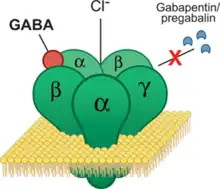
Pregabalin is a gabapentinoid and acts by inhibiting certain calcium channels.[19][20] Specifically it is a ligand of the auxiliary α2δ subunit site of certain voltage-dependent calcium channels (VDCCs), and thereby acts as an inhibitor of α2δ subunit-containing VDCCs.[19][66] There are two drug-binding α2δ subunits, α2δ-1 and α2δ-2, and pregabalin shows similar affinity for (and hence lack of selectivity between) these two sites.[19] Pregabalin is selective in its binding to the α2δ VDCC subunit.[66][67] Despite the fact that pregabalin is a GABA analogue,[68] it does not bind to the GABA receptors, does not convert into GABA or another GABA receptor agonist in vivo, and does not directly modulate GABA transport or metabolism.[20][66] However, pregabalin has been found to produce a dose-dependent increase in the brain expression of L-glutamic acid decarboxylase (GAD), the enzyme responsible for synthesizing GABA, and hence may have some indirect GABAergic effects by increasing GABA levels in the brain.[69][70][71] There is currently no evidence that the effects of pregabalin are mediated by any mechanism other than inhibition of α2δ-containing VDCCs.[66][72] In accordance, inhibition of α2δ-1-containing VDCCs by pregabalin appears to be responsible for its anticonvulsant, analgesic, and anxiolytic effects.[66][72]
The endogenous α-amino acids L-leucine and L-isoleucine, which closely resemble pregabalin and the other gabapentinoids in chemical structure, are apparent ligands of the α2δ VDCC subunit with similar affinity as the gabapentinoids (e.g., IC50 = 71 nM for L-isoleucine), and are present in human cerebrospinal fluid at micromolar concentrations (e.g., 12.9 μM for L-leucine, 4.8 μM for L-isoleucine).[73] It has been theorized that they may be the endogenous ligands of the subunit and that they may competitively antagonize the effects of gabapentinoids.[73][74] In accordance, while gabapentinoids like pregabalin and gabapentin have nanomolar affinities for the α2δ subunit, their potencies in vivo are in the low micromolar range, and competition for binding by endogenous L-amino acids has been said to likely be responsible for this discrepancy.[72]
Pregabalin was found to possess 6-fold higher affinity than gabapentin for α2δ subunit-containing VDCCs in one study.[75][76] However, another study found that pregabalin and gabapentin had similar affinities for the human recombinant α2δ-1 subunit (Ki = 32 nM and 40 nM, respectively).[77] In any case, pregabalin is 2 to 4 times more potent than gabapentin as an analgesic [68][78] and, in animals, appears to be 3 to 10 times more potent than gabapentin as an anticonvulsant.[68][78]
Pharmacokinetics
Absorption
Pregabalin is absorbed from the intestines by an active transport process mediated via the large neutral amino acid transporter 1 (LAT1, SLC7A5), a transporter for amino acids such as L-leucine and L-phenylalanine.[19][66][79] Very few (less than 10 drugs) are known to be transported by this transporter.[80] Unlike gabapentin, which is transported solely by the LAT1,[79][12] pregabalin seems to be transported not only by the LAT1 but also by other carriers.[19] The LAT1 is easily saturable, so the pharmacokinetics of gabapentin are dose-dependent, with diminished bioavailability and delayed peak levels at higher doses.[19] In contrast, this is not the case for pregabalin, which shows linear pharmacokinetics and no saturation of absorption.[19]
The oral bioavailability of pregabalin is greater than or equal to 90% across and beyond its entire clinical dose range (75 to 900 mg/day).[12] Food does not significantly influence the oral bioavailability of pregabalin.[12] Pregabalin is rapidly absorbed when administered on an empty stomach, with a Tmax (time to peak levels) of generally less than or equal to 1 hour at doses of 300 mg or less.[19][11] However, food has been found to substantially delay the absorption of pregabalin and to significantly reduce peak levels without affecting the bioavailability of the drug; Tmax values for pregabalin of 0.6 hours in a fasted state and 3.2 hours in a fed state (5-fold difference), and the Cmax is reduced by 25–31% in a fed versus fasted state.[12]
Distribution
Pregabalin crosses the blood–brain barrier and enters the central nervous system.[66] However, due to its low lipophilicity,[12] pregabalin requires active transport across the blood–brain barrier.[79][66][81][82] The LAT1 is highly expressed at the blood–brain barrier[83] and transports pregabalin across into the brain.[79][66][81][82] Pregabalin has been shown to cross the placenta in rats and is present in the milk of lactating rats.[11] In humans, the volume of distribution of an orally administered dose of pregabalin is approximately 0.56 L/kg.[11] Pregabalin is not significantly bound to plasma proteins (<1%).[12]
Metabolism
Pregabalin undergoes little or no metabolism.[12][19][84] In experiments using nuclear medicine techniques, it was revealed that approximately 98% of the radioactivity recovered in the urine was unchanged pregabalin.[11] The main metabolite is N-methylpregabalin.[11]
Elimination
Pregabalin is eliminated by the kidneys in the urine, mainly in its unchanged form.[12][11] It has a relatively short elimination half-life, with a reported value of 6.3 hours.[12] Because of its short elimination half-life, pregabalin is administered 2 to 3 times per day to maintain therapeutic levels.[12] The kidney clearance of pregabalin is 73 mL/minute.[85]
Chemistry
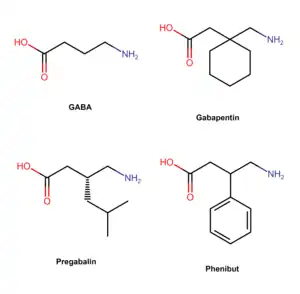
Pregabalin is a GABA analogue that is a 3-substituted derivative as well as a γ-amino acid.[86][67] Specifically, pregabalin is (S)-(+)-3-isobutyl-GABA.[87][88][89] Pregabalin also closely resembles the α-amino acids L-leucine and L-isoleucine, and this may be of greater relevance in relation to its pharmacodynamics than its structural similarity to GABA.[73][74][87]
Synthesis
Chemical syntheses of pregabalin have been described.[90][91]
History
 | |
Pregabalin was synthesized in 1990 as an anticonvulsant. It was invented by medicinal chemist Richard Bruce Silverman at Northwestern University in Evanston, Illinois.[92] Silverman is best known for identifying the drug pregabalin as a possible treatment for epileptic seizures.[93] During 1988 to 1990, Ryszard Andruszkiewicz, a visiting research fellow, synthesized a series of molecules for Silverman.[94] One looked particularly promising.[95] The molecule was effectively shaped for transportation into the brain, where it activated L-glutamic acid decarboxylase, an enzyme. Silverman hoped that the enzyme would increase production of the inhibitory neurotransmitter GABA and block convulsions.[93] Eventually, the set of molecules were sent to Parke-Davis Pharmaceuticals for testing. The drug was approved in the European Union in 2004. The US received FDA approval for use in treating epilepsy, diabetic neuropathic pain, and postherpetic neuralgia in December 2004. Pregabalin then appeared on the US market under the brand name Lyrica in fall of 2005.[96] In 2017, the U.S. Food and Drug Administration (FDA) approved pregabalin extended-release Lyrica CR, but unlike the immediate release formulation, it was not approved for the management of fibromyalgia or as add on therapy for adult with partial onset seizures.[97][98]
Society and culture
Cost
Pregabalin is available as a generic medication in a number of countries, including the United States as of July 2019.[5][22][99] In the United States as of July 2019 the wholesale/pharmacy cost for generic pregabalin is US$0.17-0.22 per 150 mg capsule.[100]
.svg.png.webp) Pregabalin costs (US)
Pregabalin costs (US).svg.png.webp) Pregabalin prescriptions (US)
Pregabalin prescriptions (US)
Legal status
- United States: During clinical trials a small number of users (~4%) reported euphoria after use, which led to its control in the US.[101] The Drug Enforcement Administration (DEA) classified pregabalin as a depressant and placed pregabalin, including its salts, and all products containing pregabalin into Schedule V of the Controlled Substances Act.[53][102][103][104]
- Norway: Pregabalin is in prescription Schedule B, alongside benzodiazepines.[105][106]
- United Kingdom: On January 14, 2016 the Advisory Council on the Misuse of Drugs (ACMD) wrote a letter to Home Office ministers recommending that pregabalin alongside gabapentin should be controlled under the Misuse of Drugs Act 1971.[107][108] It was announced in October 2018, that Pregabalin would become reclassified as a class C controlled substance from April 2019.[109][27]
Approval
In the United States, the Food and Drug Administration (FDA) has approved pregabalin for adjunctive therapy for adults with partial onset seizures, management of postherpetic neuralgia and neuropathic pain associated with spinal cord injury and diabetic peripheral neuropathy, and the treatment of fibromyalgia.[110] Pregabalin has also been approved in the European Union, the United States and Russia for treatment of generalized anxiety disorder.[99][47][111]
Marketing
Since 2008, Pfizer has engaged in extensive direct-to-consumer advertising campaigns to promote its branded product Lyrica for fibromyalgia and diabetic nerve pain indications. In January 2016, the company spent a record amount, $24.6 million for a single drug on TV ads, reaching global revenues of $14 billion, more than half in the United States.[112]
Up until 2009, Pfizer promoted Lyrica for other uses which had not been approved by medical regulators. For Lyrica and three other drugs, Pfizer was fined a record amount of US$2.3 billion by the Department of Justice,[113][114][115] after pleading guilty to advertising and branding "with the intent to defraud or mislead." Pfizer illegally promoted the drugs, with doctors "invited to consultant meetings, many in resort locations; attendees expenses were paid; they received a fee just for being there", according to prosecutor Michael Loucks.[113][114]
Intellectual property
Professor Richard "Rick" Silverman of Northwestern University developed pregabalin there. The university holds a patent on it, exclusively licensed to Pfizer.[116][117] That patent, along with others, was challenged by generic manufacturers and was upheld in 2014, giving Pfizer exclusivity for Lyrica in the US until 2018.[118][119]
As of October 2017, pregabalin was marketed under many brand names in other countries: Algerika, Alivax, Alyse, Alzain, Andogablin, Aprion, Averopreg, Axual, Balifibro, Brieka, Clasica, Convugabalin, Dapapalin, Dismedox, Dolgenal, Dolica, Dragonor, Ecubalin, Epica, Epiron, Gaba-P, Gabanext, Gabarol, Gabica, Gablin, Gablovac, Gabrika, Gavin, Gialtyn, Glonervya, Helimon, Hexgabalin, Irenypathic, Kabian, Kemirica, Kineptia, Lecaent, Lingabat, Linprel, Lyric, Lyrica, Lyrineur, Lyrolin, Martesia, Maxgalin, Mystika, Neuragabalin, Neugaba, Neurega, Neurica, Neuristan, Neurolin, Neurovan, Neurum, Newrica, Nuramed, Paden, Pagadin, Pagamax, Painica, Pevesca, PG, Plenica, Pragiola, Prebalin, Prebanal, Prebel, Prebictal, Prebien, Prefaxil, Pregaba, Pregabalin, Pregabalina, Pregabaline, Prégabaline, Pregabalinum, Pregabateg, Pregaben, Pregabid, Pregabin, Pregacent, Pregadel, Pregagamma, Pregalex, Pregalin, Pregamid, Pregan, Preganerve, Pregastar, Pregatrend, Pregavalex, Pregdin Apex, Pregeb, Pregobin, Prejunate, Prelin, Preludyo, Prelyx, Premilin, Preneurolin, Prestat, Pretor, Priga, Provelyn, Regapen, Resenz, Rewisca, Serigabtin, Symra, Vronogabic, Xablin, and Xil.[120]
It was also marketed in several countries as a combination drug with mecobalamin under the brand names Agemax-P, Alphamix-PG, Freenerve-P, Gaben, Macraberin-P, Mecoblend-P, Mecozen-PG, Meex-PG, Methylnuron-P, Nervolin, Nervopreg, Neurica-M, Neuroprime-PG, Neutron-OD, Nuroday-P, Nurodon-PG, Nuwin-P, Pecomin-PG, Prebel-M, Predic-GM, Pregacent-M, Pregamet, Preganerv-M, Pregeb-M OD, Pregmic, Prejunate Plus, Preneurolin Plus, Pretek-GM, Rejusite, Renerve-P, Safyvit-PR, and Vitcobin-P, Voltanerv with Methylcobalamin and ALA by Cogentrix Pharma.[120]
Pfizer's main patent for Lyrica, for seizure disorders, in the UK expired in 2013. In November 2018 the Supreme Court of the United Kingdom ruled that Pfizer's second patent on the drug, for treatment of pain, was invalid because there was a lack of evidence for the conditions it covered – central and peripheral neuropathic pain. From October 2015 GPs were forced to change people from generic pregabalin to branded until the second patent ran out in July 2017. This cost the NHS £502 million.[121]
References
- ↑ "Pregabalin - Drugs.com". www.drugs.com. Archived from the original on August 28, 2019. Retrieved November 7, 2016.
- 1 2 3 4 5 6 7 8 9 "Pregabalin". The American Society of Health-System Pharmacists. Archived from the original on December 2, 2019. Retrieved February 3, 2019.
- 1 2 Frampton, James E. (2014). "Pregabalin: A Review of its Use in Adults with Generalized Anxiety Disorder". CNS Drugs. 28 (9): 835–54. doi:10.1007/s40263-014-0192-0. PMID 25149863.
- 1 2 Iftikhar, IH; Alghothani, L; Trotti, LM (December 2017). "Gabapentin enacarbil, pregabalin and rotigotine are equally effective in restless legs syndrome: a comparative meta-analysis". European Journal of Neurology. 24 (12): 1446–1456. doi:10.1111/ene.13449. PMID 28888061.
- 1 2 3 4 5 British National Formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 323. ISBN 9780857113382.
- 1 2 3 4 Schifano, Fabrizio (2014). "Misuse and Abuse of Pregabalin and Gabapentin: Cause for Concern?". CNS Drugs. 28 (6): 491–6. doi:10.1007/s40263-014-0164-4. PMID 24760436.
- 1 2 "Pregabalin Use During Pregnancy". Drugs.com. June 12, 2018. Archived from the original on March 21, 2019. Retrieved January 21, 2020.
- ↑ "Pregabalin (Professional Patient Advice) - Drugs.com". www.drugs.com. Archived from the original on July 31, 2020. Retrieved November 7, 2016.
- ↑ Lilley, Linda Lane; Collins, Shelly Rainforth; Snyder, Julie S. (2015). Pharmacology and the Nursing Process. Elsevier Health Sciences. p. 227. ISBN 9780323358286.
- 1 2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on November 17, 2020. Retrieved September 9, 2020.
- 1 2 3 4 5 6 7 8 "Summary of product characteristics" (PDF). European Medicines Agency. March 6, 2013. Archived (PDF) from the original on September 16, 2018. Retrieved May 6, 2013.
- 1 2 3 4 5 6 7 8 9 10 11 Bockbrader, H. N.; Wesche, D.; Miller, R.; Chapel, S.; Janiczek, N.; Burger, P. (2010). "A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin". Clinical Pharmacokinetics. 49 (10): 661–669. doi:10.2165/11536200-000000000-00000. PMID 20818832.
{{cite journal}}: CS1 maint: uses authors parameter (link) - ↑ "Pregabalin (Professional Patient Advice)". Drugs.com. Archived from the original on July 31, 2020. Retrieved August 4, 2020.
- ↑ Hantson, P; Courtois, F; Borrey, D; Haufroid, V (2014). "Pregabalin-associated myoclonic encephalopathy without evidence of drug accumulation in a patient with acute renal failure". Indian Journal of Nephrology. 24 (1): 48–50. doi:10.4103/0971-4065.125102. PMC 3927193. PMID 24574633.
- ↑ Randinitis, Edward J.; Posvar, Edward L.; Alvey, Christine W.; Sedman, Allen J.; Cook, Jack A.; Bockbrader, Howard N. (2003). "Pharmacokinetics of Pregabalin in Subjects with Various Degrees of Renal Function". The Journal of Clinical Pharmacology. 43 (3): 277–83. doi:10.1177/0091270003251119. PMID 12638396.
- ↑ Mishriky, B. M.; Waldron, N. H.; Habib, A. S. (January 2015). "Impact of pregabalin on acute and persistent postoperative pain: a systematic review and meta-analysis". British Journal of Anaesthesia. 114 (1): 10–31. doi:10.1093/bja/aeu293. ISSN 1471-6771. PMID 25209095.
- ↑ "4. Nervous system". BNF (84 ed.). London: BMJ Group and the Pharmaceutical Press. September 2022 – March 2023. pp. 353–355. ISBN 978-0-85711-432-7.
- ↑ "Pregabalin Use During Pregnancy". Drugs.com. Archived from the original on March 21, 2019. Retrieved February 4, 2019.
- 1 2 3 4 5 6 7 8 9 10 Calandre, E. P.; Rico-Villademoros, F.; Slim, M. (2016). "Alpha2delta ligands, gabapentin, pregabalin and mirogabalin: a review of their clinical pharmacology and therapeutic use". Expert Review of Neurotherapeutics. 16 (11): 1263–1277. doi:10.1080/14737175.2016.1202764. PMID 27345098.
{{cite journal}}: CS1 maint: uses authors parameter (link) - 1 2 3 Uchitel, O. D.; Di Guilmi, M. N.; Urbano, F. J.; Gonzalez-Inchauspe, C. (2010). "Acute modulation of calcium currents and synaptic transmission by gabapentinoids". Channels (Austin). 4 (6): 490–496. doi:10.4161/chan.4.6.12864. PMID 21150315.
{{cite journal}}: CS1 maint: uses authors parameter (link) - ↑ Kaye, Alan David; Vadivelu, Nalini; Urman, Richard D. (2014). Substance Abuse: Inpatient and Outpatient Management for Every Clinician. Springer. p. 324. ISBN 9781493919512. Archived from the original on March 27, 2019. Retrieved August 4, 2020.
- 1 2 "Generic Lyrica Availability". Drugs.com. Archived from the original on March 27, 2019. Retrieved February 4, 2019.
- ↑ "FDA approves first generics of Lyrica". U.S. Food and Drug Administration (FDA) (Press release). September 11, 2019. Archived from the original on June 9, 2020. Retrieved October 27, 2019.
- ↑ "NADAC as of 2019-10-23". Centers for Medicare and Medicaid Services. Archived from the original on October 27, 2019. Retrieved October 27, 2019.
- ↑ "The Top 300 of 2020". ClinCalc. Archived from the original on March 18, 2020. Retrieved April 2, 2020.
- ↑ "Pregabalin". ClinCalc. December 23, 2019. Archived from the original on July 8, 2020. Retrieved April 2, 2020.
- 1 2 "Pregabalin and gabapentin to be controlled as Class C drugs". GOV.UK. October 15, 2018. Archived from the original on November 12, 2020. Retrieved April 2, 2020.
- ↑ Moisset, X; Bouhassira, D; Attal, N (September 2021). "French guidelines for neuropathic pain: An update and commentary". Revue neurologique. 177 (7): 834–837. doi:10.1016/j.neurol.2021.07.004. PMID 34332778.
- ↑ Panebianco M, Bresnahan R, Hemming K, Marson AG (July 2019). "Pregabalin add-on for drug-resistant focal epilepsy". Cochrane Database of Systematic Reviews. 7: CD005612. doi:10.1002/14651858.CD005612.pub4. PMC 6614921. PMID 31287157.
- 1 2 Zhou Q, Zheng J, Yu L, Jia X (October 2012). "Pregabalin monotherapy for epilepsy". Cochrane Database of Systematic Reviews. 10: CD009429. doi:10.1002/14651858.CD009429.pub2. PMID 23076957.
- ↑ Attal, N.; Cruccu, G.; Baron, R.; et al. (September 2010). "EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision". European Journal of Neurology. 17 (9): 1113–e88. doi:10.1111/j.1468-1331.2010.02999.x. PMID 20402746. Archived from the original on August 29, 2021. Retrieved August 4, 2020.
- 1 2 Derry S, Bell RF, Straube S, Wiffen PJ, Aldington D, Moore RA (January 2019). "Pregabalin for neuropathic pain in adults". Cochrane Database of Systematic Reviews. 1: CD007076. doi:10.1002/14651858.CD007076.pub3. PMC 6353204. PMID 30673120.
- ↑ Finnerup, N.B.; Sindrup, S. H.; Jensen, T. S. (September 2010). "The evidence for pharmacological treatment of neuropathic pain". Pain. 150 (3): 573–81. doi:10.1016/j.pain.2010.06.019. PMID 20705215.
- ↑ Enke, O.; New, H. A.; New, C. H.; Mathieson, S.; McLachlan, A. J.; Latimer, J.; Maher, C. G.; Lin, C. C. (July 3, 2018). "Anticonvulsants in the treatment of low back pain and lumbar radicular pain: a systematic review and meta-analysis". Canadian Medical Association Journal. 190 (26): E786–E793. doi:10.1503/cmaj.171333. PMC 6028270. PMID 29970367.
- ↑ Bennett, Michael I.; Laird, Barry; van Litsenburg, Chantal; Nimour, Meryem (2013). "Pregabalin for the Management of Neuropathic Pain in Adults with Cancer: A Systematic Review of the Literature". Pain Medicine. 14 (11): 1681–8. doi:10.1111/pme.12212. PMID 23915361.
- ↑ Howard, Paul (2017). Palliative care formulary (6 ed.). Pharmaceutical press. p. Chapter 4 Central Nervous System. ISBN 978-0-85711-348-1.
- ↑ Linde M, Mulleners WM, Chronicle EP, McCrory DC (June 2013). "Gabapentin or pregabalin for the prophylaxis of episodic migraine in adults". Cochrane Database of Systematic Reviews. 6 (6): CD010609. doi:10.1002/14651858.CD010609. PMC 6599858. PMID 23797675.
- 1 2 Clarke, H.; Bonin, R. P.; Orser, B. A.; Englesakis, M.; Wijeysundera, D. N.; Katz, J. (August 2012). "The prevention of chronic postsurgical pain using gabapentin and pregabalin: a combined systematic review and meta-analysis". Anesthesia & Analgesia. 115 (2): 428–42. doi:10.1213/ANE.0b013e318249d36e. hdl:10315/27968. PMID 22415535.
{{cite journal}}: CS1 maint: uses authors parameter (link) - ↑ Chaparro LE, Smith SA, Moore RA, Wiffen PJ, Gilron I (July 2013). "Pharmacotherapy for the prevention of chronic pain after surgery in adults". Cochrane Database of Systematic Reviews (7): CD008307. doi:10.1002/14651858.CD008307.pub2. PMC 6481826. PMID 23881791.
- ↑ Hamilton, T. W.; Strickland, L. H.; Pandit, H. G. (August 17, 2016). "A Meta-Analysis on the Use of Gabapentinoids for the Treatment of Acute Postoperative Pain Following Total Knee Arthroplasty". The Journal of Bone and Joint Surgery, American Volume. 98 (16): 1340–50. doi:10.2106/jbjs.15.01202. PMID 27535436.
- ↑ Patel, Ryan; Dickenson, Anthony H. (2016). "Mechanisms of the gabapentinoids and α2δ-1 calcium channel subunit in neuropathic pain". Pharmacology Research & Perspectives. 4 (2): e00205. doi:10.1002/prp2.205. PMC 4804325. PMID 27069626.
- ↑ Slee A, Nazareth I, Bondaronek P, Liu Y, Cheng Z, Freemantle N (February 2019). "Pharmacological treatments for generalised anxiety disorder: a systematic review and network meta-analysis". Lancet. 393 (10173): 768–777. doi:10.1016/S0140-6736(18)31793-8. PMID 30712879.
- ↑ Ton, Joey (July 16, 2018). "#216 Anxiously Awaiting Evidence: Pregabalin in generalized anxiety disorder". CFPCLearn. Archived from the original on January 30, 2023. Retrieved June 16, 2023.
- ↑ Wensel, T. M.; Powe, K. W.; Cates, M. E. (March 2012). "Pregabalin for the treatment of generalized anxiety disorder". Annals of Pharmacotherapy. 46 (3): 424–9. doi:10.1345/aph.1Q405. PMID 22395254.
{{cite journal}}: CS1 maint: uses authors parameter (link) - ↑ Owen, Richard T. (September 2007). "Pregabalin: its efficacy, safety and tolerability profile in generalized anxiety". Drugs of Today. 43 (9): 601–10. doi:10.1358/dot.2007.43.9.1133188. PMID 17940637.
- ↑ Bandelow, B.; Sher, L.; Bunevicius, R.; et al. (June 2012). "Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care". International Journal of Psychiatry in Clinical Practice. 16 (2): 77–84. doi:10.3109/13651501.2012.667114. PMID 22540422. Archived from the original on August 29, 2021. Retrieved August 4, 2020.
- 1 2 3 4 Bandelow, Borwin; Wedekind, Dirk; Leon, Teresa (2007). "Pregabalin for the treatment of generalized anxiety disorder: a novel pharmacologic intervention". Expert Review of Neurotherapeutics. 7 (7): 769–81. doi:10.1586/14737175.7.7.769. PMID 17610384.
- ↑ Owen, R. T. (2007). "Pregabalin: Its efficacy, safety and tolerability profile in generalized anxiety". Drugs of Today. 43 (9): 601–10. doi:10.1358/dot.2007.43.9.1133188. PMID 17940637.
- ↑ Shanthanna, Harsha; Gilron, Ian; Rajarathinam, Manikandan; AlAmri, Rizq; Kamath, Sriganesh; Thabane, Lehana; Devereaux, Philip J.; Bhandari, Mohit; Tsai, Alexander C. (August 15, 2017). "Benefits and safety of gabapentinoids in chronic low back pain: A systematic review and meta-analysis of randomized controlled trials". PLOS Medicine. 14 (8): e1002369. doi:10.1371/journal.pmed.1002369. PMC 5557428. PMID 28809936.
- ↑ Mannix, Liam (December 18, 2018). "This popular drug is linked to addiction and suicide. Why do doctors keep prescribing it?". The Canberra Times. Archived from the original on December 18, 2018. Retrieved December 18, 2018.
- ↑ Freynhagen, R.; Backonja, M.; Schug, S.; Lyndon, G.; Parsons, B.; Watt, S.; Behar, R. (December 2016). "Pregabalin for the Treatment of Drug and Alcohol Withdrawal Symptoms: A Comprehensive Review". CNS Drugs. 30 (12): 1191–1200. doi:10.1007/s40263-016-0390-z. PMC 5124051. PMID 27848217.
- ↑ Onakpoya IJ, Thomas ET, Lee JJ, Goldacre B, Heneghan CJ (January 2019). "Benefits and harms of pregabalin in the management of neuropathic pain: a rapid review and meta-analysis of randomised clinical trials". BMJ Open. 9 (1): e023600. doi:10.1136/bmjopen-2018-023600. PMC 6347863. PMID 30670513.
- 1 2 3 Drug Enforcement Administration, Department of Justice (July 2005). "Schedules of controlled substances: placement of pregabalin into schedule V. Final rule". Federal Register. 70 (144): 43633–5. PMID 16050051. Archived from the original on December 31, 2011. Retrieved January 22, 2012.
- ↑ Pfizer Australia Pty Ltd. Lyrica (Australian Approved Product Information). West Ryde: Pfizer, 2006
- ↑ Rossi, Simone, ed. (2006). Australian Medicines Handbook, 2006. Australian Medicines Handbook. ISBN 978-0-9757919-2-9.
- ↑ "Medication Guide (Pfizer Inc.)" (PDF). U.S. Food and Drug Administration (FDA). June 2011. Archived (PDF) from the original on September 8, 2011. Retrieved November 6, 2011.
- ↑ Millar, J; Sadasivan, S; Weatherup, N; Lutton, S (September 7, 2013). "Lyrica Nights–Recreational Pregabalin Abuse in an Urban Emergency Department". Emergency Medicine Journal. 30 (10): 874.2–874. doi:10.1136/emermed-2013-203113.20.
- ↑ "Lyrica Capsules". medicines.org.uk. Archived from the original on October 20, 2020. Retrieved August 4, 2020.
- ↑ "Pregabalin Pregnancy and Breastfeeding Warnings". Archived from the original on March 21, 2019. Retrieved August 29, 2016.
- 1 2 3 4 5 6 7 8 9 "FDA warns about serious breathing problems with seizure and nerve pain medicines gabapentin (Neurontin, Gralise, Horizant) and pregabalin (Lyrica, Lyrica CR)". U.S. Food and Drug Administration (FDA). December 19, 2019. Archived from the original on December 22, 2019. Retrieved December 21, 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ "FDA warns about serious breathing problems with seizure and nerve pain medicines gabapentin (Neurontin, Gralise, Horizant) and pregabalin (Lyrica, Lyrica CR) When used with CNS depressants or in patients with lung problems" (PDF). December 19, 2019. Archived from the original on December 22, 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ Murphy, N. G.; Mosher, L. (2008). "Severe myoclonus from pregabalin (Lyrica) due to chronic renal insufficiency". Clinical Toxicology. 46 (7): 594. doi:10.1080/15563650802255033.
- ↑ Yoo, Lawrence; Matalon, Daniel; Hoffman, Robert S.; Goldfarb, David S. (2009). "Treatment of pregabalin toxicity by hemodialysis in a patient with kidney failure". American Journal of Kidney Diseases. 54 (6): 1127–30. doi:10.1053/j.ajkd.2009.04.014. PMID 19493601.
- ↑ Baselt, Randall C. (2008). Disposition of Toxic Drugs and Chemicals in Man (8th ed.). Biomedical Publications. pp. 1296–1297. ISBN 978-0-9626523-7-0.
- ↑ Pregabalin. In: Lexi-Drugs [database on the Internet]. Hudson (OH): Lexi-Comp, Inc.; 2007 [cited 2015 Oct 29].
- 1 2 3 4 5 6 7 8 9 Sills, G. J. (2006). "The mechanisms of action of gabapentin and pregabalin". Current Opinion in Pharmacology. 6 (1): 108–13. doi:10.1016/j.coph.2005.11.003. PMID 16376147.
{{cite journal}}: CS1 maint: uses authors parameter (link) - 1 2 Benzon, Honorio; Rathmell, James P.; Wu, Christopher L.; Turk, Dennis C.; Argoff, Charles E.; Hurley, Robert W. (September 11, 2013). Practical Management of Pain. Elsevier Health Sciences. p. 1006. ISBN 978-0-323-17080-2.
- 1 2 3 Bryans, Justin S.; Wustrow, David J. (1999). "3-Substituted GABA analogs with central nervous system activity: A review". Medicinal Research Reviews. 19 (2): 149–77. doi:10.1002/(SICI)1098-1128(199903)19:2<149::AID-MED3>3.0.CO;2-B. PMID 10189176.
- ↑ Lin, Guo-Qiang; You, Qi-Dong; Cheng, Jie-Fei (August 8, 2011). "Chiral Drugs: Chemistry and Biological Action". John Wiley & Sons – via Google Books.
- ↑ "Pregabalin". Archived from the original on September 16, 2018. Retrieved August 4, 2020.
- ↑ Sze, P. Y. (1979). L-Glutamate decarboxylase. Advances in Experimental Medicine and Biology. Vol. 123. pp. 59–78. doi:10.1007/978-1-4899-5199-1_4. ISBN 978-1-4899-5201-1. PMID 390996.
- 1 2 3 Stahl, S. M.; Porreca, F.; Taylor, C. P.; Cheung, R.; Thorpe, A. J.; Clair, A. (2013). "The diverse therapeutic actions of pregabalin: is a single mechanism responsible for several pharmacological activities?". Trends in Pharmacological Sciences. 34 (6): 332–9. doi:10.1016/j.tips.2013.04.001. PMID 23642658.
{{cite journal}}: CS1 maint: uses authors parameter (link) - 1 2 3 Dooley, D. J.; Taylor, C. P.; Donevan, S.; Feltner, D. (2007). "Ca2+ channel alpha2delta ligands: novel modulators of neurotransmission". Trends in Pharmacological Sciences. 28 (2): 75–82. doi:10.1016/j.tips.2006.12.006. PMID 17222465.
{{cite journal}}: CS1 maint: uses authors parameter (link) - 1 2 Davies, A.; Hendrich, J.; Van Minh, A. T.; Wratten, J.; Douglas, L.; Dolphin, A. C. (2007). "Functional biology of the alpha(2)delta subunits of voltage-gated calcium channels". Trends in Pharmacological Sciences. 28 (5): 220–228. doi:10.1016/j.tips.2007.03.005. PMID 17403543.
{{cite journal}}: CS1 maint: uses authors parameter (link) - ↑ Arora, Mahesh Kumar; Agarwal, Anil; Baidya, Dalim Kumar; Khanna, Puneet (2011). "Pregabalin in acute and chronic pain". Journal of Anaesthesiology Clinical Pharmacology. 27 (3): 307–14. doi:10.4103/0970-9185.83672. PMC 3161452. PMID 21897498.
- ↑ McMahon, Stephen B. (2013). Wall and Melzack's textbook of pain (6th ed.). Philadelphia, PA: Elsevier/Saunders. p. 515. ISBN 9780702040597.
- ↑ Taylor, C. P.; Angelotti, T.; Fauman, E. (February 2007). "Pharmacology and mechanism of action of pregabalin: the calcium channel alpha2-delta (alpha2-delta) subunit as a target for antiepileptic drug discovery". Epilepsy Research. 73 (2): 137–150. doi:10.1016/j.eplepsyres.2006.09.008. PMID 17126531.
{{cite journal}}: CS1 maint: uses authors parameter (link) - 1 2 Lauria-Horner, Bianca A.; Pohl, Robert B. (2003). "Pregabalin: a new anxiolytic". Expert Opinion on Investigational Drugs. 12 (4): 663–72. doi:10.1517/13543784.12.4.663. PMID 12665421.
- 1 2 3 4 Dickens, D.; Webb, S. D.; Antonyuk, S.; Giannoudis, A.; Owen, A.; Rädisch, S.; Hasnain, S. S.; Pirmohamed, M. (2013). "Transport of gabapentin by LAT1 (SLC7A5)". Biochemical Pharmacology. 85 (11): 1672–1683. doi:10.1016/j.bcp.2013.03.022. PMID 23567998.
{{cite journal}}: CS1 maint: uses authors parameter (link) - ↑ del Amo, E. M.; Urtti, A.; Yliperttula, M. (2008). "Pharmacokinetic role of L-type amino acid transporters LAT1 and LAT2". European Journal of Pharmaceutical Sciences. 35 (3): 161–174. doi:10.1016/j.ejps.2008.06.015. PMID 18656534.
{{cite journal}}: CS1 maint: uses authors parameter (link) - 1 2 Geldenhuys, W. J.; Mohammad, A. S.; Adkins, C. E.; Lockman, P. R. (2015). "Molecular determinants of blood-brain barrier permeation". Therapeutic Delivery. 6 (8): 961–971. doi:10.4155/tde.15.32. PMC 4675962. PMID 26305616.
{{cite journal}}: CS1 maint: uses authors parameter (link) - 1 2 Müller, C. E. (2009). "Prodrug approaches for enhancing the bioavailability of drugs with low solubility". Chemistry & Biodiversity. 6 (11): 2071–2083. doi:10.1002/cbdv.200900114. PMID 19937841.
{{cite journal}}: CS1 maint: uses authors parameter (link) - ↑ Boado, R. J.; Li, J. Y.; Nagaya, M.; Zhang, C.; Pardridge, W. M. (1999). "Selective expression of the large neutral amino acid transporter at the blood-brain barrier". Proceedings of the National Academy of Sciences USA. 96 (21): 12079–12084. Bibcode:1999PNAS...9612079B. doi:10.1073/pnas.96.21.12079. PMC 18415. PMID 10518579.
{{cite journal}}: CS1 maint: uses authors parameter (link) - ↑ McElroy, Susan L.; Keck, Paul E.; Post, Robert M., eds. (2008). Antiepileptic Drugs to Treat Psychiatric Disorders. INFRMA-HC. p. 370. ISBN 978-0-8493-8259-8.
- ↑ "LYRICA – pregabalin capsule". DailyMed. U.S. National Library of Medicine. September 2010. Archived from the original on April 2, 2015. Retrieved May 6, 2013.
- ↑ Wyllie, Elaine; Cascino, Gregory D.; Gidal, Barry E.; Goodkin, Howard P. (February 17, 2012). Wyllie's Treatment of Epilepsy: Principles and Practice. Lippincott Williams & Wilkins. p. 423. ISBN 978-1-4511-5348-4.
- 1 2 Yogeeswari, P.; Ragavendran, J. V.; Sriram, D. (2006). "An update on GABA analogs for CNS drug discovery". Recent Patents on CNS Drug Discovery. 1 (1): 113–118. doi:10.2174/157488906775245291. PMID 18221197.
{{cite journal}}: CS1 maint: uses authors parameter (link) - ↑ Rose, M. A.; Kam, P. C. (2002). "Gabapentin: pharmacology and its use in pain management". Anaesthesia. 57 (5): 451–462. doi:10.1046/j.0003-2409.2001.02399.x. PMID 11966555.
{{cite journal}}: CS1 maint: uses authors parameter (link) - ↑ Wheless, James W.; Willmore, James; Brumback, Roger A. (2009). Advanced Therapy in Epilepsy. PMPH-USA. p. 302. ISBN 978-1-60795-004-2.
- ↑ Vardanyan, Ruben; Hruby, Victor (January 7, 2016). Synthesis of Best-Seller Drugs. Elsevier Science. p. 158. ISBN 978-0-12-411524-8. Archived from the original on August 29, 2021. Retrieved August 4, 2020.
- ↑ Andrushko, Vasyl; Andrushko, Natalia (August 16, 2013). Stereoselective Synthesis of Drugs and Natural Products. John Wiley & Sons. p. 869. ISBN 978-1-118-62833-1. Archived from the original on August 29, 2021. Retrieved August 4, 2020.
- ↑ Lowe, Derek (March 25, 2008). "Getting to Lyrica". In the Pipeline. Science. Archived from the original on November 21, 2015. Retrieved November 21, 2015.
- 1 2 Merrill, Nick (February 25, 2010). "Silverman's golden drug makes him NU's golden ticket". North by Northwestern. Archived from the original on June 1, 2016. Retrieved May 19, 2016.
- ↑ Andruszkiewicz, Ryszard; Silverman, Richard B. (1990). "4-Amino-3-alkylbutanoic acids as substrates for gamma-aminobutyric acid aminotransferase". The Journal of Biological Chemistry. 265 (36): 22288–91. PMID 2266125. Archived from the original on August 27, 2022. Retrieved August 4, 2020.
- ↑ Poros, Joanna (2005). "Polish scientist is the co-author of a new anti-epileptic drug". Science and Scholarship in Poland. Archived from the original on August 20, 2016. Retrieved May 19, 2016.
- ↑ Dworkin, Robert H.; Kirkpatrick, Peter (2005). "Pregabalin". Nature Reviews Drug Discovery. 4 (6): 455–6. doi:10.1038/nrd1756. PMID 15959952.
- ↑ Editorial, Reuters. "BRIEF-FDA approves Pfizer's Lyrica CR extended-release tablets CV". U.S. Archived from the original on October 4, 2018. Retrieved October 3, 2018.
- ↑ "LYRICA CR- pregabalin tablet, film coated, extended release PI". labeling.pfizer.com. Archived from the original on October 20, 2020. Retrieved October 27, 2019.
- 1 2 Levy, Sandra. Nine generic firms get FDA approval for generic Lyrica. Archived August 5, 2020, at the Wayback Machine Drug Store News, July 22, 2019.
- ↑ "Generic Lyrica launches at 97% discount to brand version". 46brooklyn Research. Archived from the original on July 16, 2020. Retrieved August 4, 2020.
- ↑ "Lyrica - FDA prescribing information, side effects and uses". Drugs.com. Archived from the original on August 13, 2017. Retrieved August 4, 2020.
- ↑ Drug Enforcement Administration, Department of Justice (July 2005). "Schedules of controlled substances: placement of pregabalin into schedule V. Final rule". Federal Register. 70 (144): 43633–43635. PMID 16050051.
- ↑ "Title 21 CFR – PART 1308 – Section 1308.15 Schedule V". usdoj.gov. Archived from the original on September 3, 2020. Retrieved August 4, 2020.
- ↑ "Placement of Pregabalin into Schedule V". Archived from the original on December 31, 2011. Retrieved August 4, 2020.
- ↑ Felleskatalogen (May 7, 2015). "Lyrica". felleskatalogen.no. Archived from the original on August 9, 2020. Retrieved August 4, 2020.
- ↑ Chalabianloo, F.; Schjøtt, J. (January 2009). "Pregabalin and its potential for abuse". Journal of the Norwegian Medical Association. 129 (3): 186–187. doi:10.4045/tidsskr.08.0047. PMID 19180163.
- ↑ "Re: Pregabalin and Gabapentin advice" (PDF). GOV.UK. January 14, 2016. Archived (PDF) from the original on November 8, 2020. Retrieved August 4, 2020.
- ↑ "Pregabalin and gabapentin: proposal to schedule under the Misuse of Drugs Regulations 2001". GOV.UK. November 10, 2017. Archived from the original on January 3, 2020. Retrieved April 2, 2020.
- ↑ Mayor, Susan (October 16, 2018). "Pregabalin and gabapentin become controlled drugs to cut deaths from misuse". British Medical Journal. 363: k4364. doi:10.1136/bmj.k4364. ISSN 0959-8138. PMID 30327316. Archived from the original on August 9, 2019. Retrieved August 4, 2020.
- ↑ "Pfizer to pay $2.3 billion to resolve criminal and civil health care liability relating to fraudulent marketing and the payment of kickbacks". Stop Medicare Fraud, U.S. Departments of Health & Human Services, and of Justice. Archived from the original on August 30, 2012. Retrieved July 4, 2012.
- ↑ "Pfizer's Lyrica Approved for the Treatment of Generalized Anxiety Disorder (GAD) in Europe" (Press release). Archived from the original on September 29, 2007. Retrieved November 6, 2011.
- ↑ Bulik, B. Snyder (March 2016), "AbbVie's Humira, Pfizer's Lyrica kick off 2016 with hefty TV ad spend" Archived March 6, 2017, at the Wayback Machine, FiercePharma, retrieved March 5, 2017
- 1 2 Harris, Gardiner (September 2, 2009). "Pfizer Pays $2.3 Billion to Settle Marketing Case". New York Times. Archived from the original on August 22, 2011. Retrieved December 21, 2017.
- 1 2 "Pfizer agrees record fraud fine". BBC News. September 2, 2009. Archived from the original on September 8, 2009. Retrieved December 21, 2017.
- ↑ "Portions of the Pfizer Inc. 2010 Financial Report". U.S. Securities and Exchange Commission. 2010. Archived from the original on June 10, 2019. Retrieved December 21, 2017.
- ↑ Jacoby, M. (2008). "Financial Windfall from Lyrica". Chemical & Engineering News. 86 (10): 56–61. doi:10.1021/cen-v086n010.p056.
- ↑ "Gamma amino butyric acid analogs and optical isomers". Archived from the original on March 21, 2019. Retrieved August 4, 2020.
- ↑ Decker, Susan; "Pfizer Wins Ruling to Block Generic Lyrica Until 2018 Archived January 8, 2015, at the Wayback Machine", Bloomberg News, February 6, 2014
- ↑ "Decision: Pfizer Inc. (PFE) v. Teva Pharmaceuticals USA Inc., 12-1576, U.S. Court of Appeals for the Federal Circuit (Washington)" (PDF). Archived (PDF) from the original on May 9, 2016. Retrieved August 4, 2020.
- 1 2 "Pregabalin international brands". Drugs.com. Archived from the original on August 28, 2019. Retrieved October 27, 2017.
- ↑ "Pfizer's failed pregabalin patent appeal means NHS could reclaim £502m". Pulse. November 14, 2018. Archived from the original on November 15, 2018. Retrieved November 15, 2018.
External links
| External sites: |
|
|---|---|
| Identifiers: |