Bevantolol
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
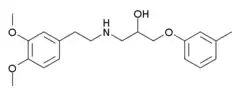
| Formula | C20H27NO4 |
| Molar mass | 345.439 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
SMILES
| |
InChI
| |
| (verify) | |
Bevantolol (INN) was a drug candidate for angina and hypertension that acted as both a beta blocker and a calcium channel blocker.[1][2] It was discovered and developed by Warner-Lambert[3] but in January 1989 the company announced that it had withdrawn the New Drug Application; the company's chairman said: "Who needs the 30th beta blocker?"[4] As of 2016 it wasn't marketed in the US, UK, or Europe and the authors of a Cochrane review could find no product monograph for it.[5]
References
- ↑ Frishman WH, Goldberg RJ, Benfield P (January 1988). "Bevantolol. A preliminary review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in hypertension and angina pectoris". Drugs. 35 (1): 1–21. doi:10.2165/00003495-198835010-00001. PMID 2894292.
- ↑ Vaughan Williams EM (July 1987). "Bevantolol: a beta-1 adrenoceptor antagonist with unique additional actions". Journal of Clinical Pharmacology. 27 (7): 450–60. doi:10.1002/j.1552-4604.1987.tb03049.x. PMID 2888789.
- ↑ McPherson EM (2007). Pharmaceutical Manufacturing Encyclopedia (3rd ed.). Burlington: Elsevier. pp. 618–619. ISBN 9780815518563.
- ↑ "Warner-Lambert Pipeline Narrowed to 40 Active Research Compounds". Pink Sheet. 30 January 1989.
- ↑ Wong GW, Boyda HN, Wright JM (March 2016). "Blood pressure lowering efficacy of beta-1 selective beta blockers for primary hypertension". The Cochrane Database of Systematic Reviews. 3: CD007451. doi:10.1002/14651858.CD007451.pub2. PMC 6486283. PMID 26961574.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.