Metipranolol
 | |
| Clinical data | |
|---|---|
| Trade names | Optipranolol |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a601078 |
| ATC code | |
| Identifiers | |
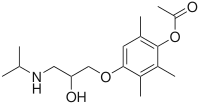
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.041.031 |
| Chemical and physical data | |
| Formula | C17H27NO4 |
| Molar mass | 309.406 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
SMILES
| |
InChI
| |
| (verify) | |
Metipranolol (OptiPranolol, Betanol, Disorat, Trimepranol) is a non-selective beta blocker used in eye drops to treat glaucoma. It is rapidly metabolized into desacetylmetipranolol.[1]
References
- ↑ Maffei Facino R, Bertuletti R, Carini M, Tofanetti O (1980). "In vitro metabolism of methypranolol by rat liver". Analytical Chemistry Symposia Series. 4 (6): 217–223.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.