Netarsudil
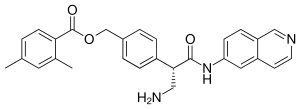 | |
| Names | |
|---|---|
| Pronunciation | ne TAR soo dil |
| Trade names | Rhopressa, Rhokiinsa |
| Other names | AR-11324 |
IUPAC name
| |
| Clinical data | |
| Drug class | Rho kinase inhibitor[1] |
| Main uses | Increased intraocular pressure, open-angle glaucoma[2][1] |
| Side effects | Red eyes, conjunctival bleeding, blurry vision, tearing[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Routes of use | Eye drops, topical |
| Duration of action | ≥ 24 hrs |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a618014 |
| Legal | |
| License data |
|
| Legal status | |
| Pharmacokinetics | |
| Metabolism | Esterases in the cornea |
| Metabolites | AR-13503 (active metabolite) |
| Elimination half-life | 16–17 hrs |
| Chemical and physical data | |
| Formula | C28H27N3O3 |
| Molar mass | 453.542 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Netarsudil, sold under the brand name Rhopressa and Rhokiinsa, is a medication used to treat increased intraocular pressure including open-angle glaucoma.[2][1] It is used as an eye drop.[2]
Common side effects include red eyes, conjunctival bleeding, blurry vision, and tearing.[1] Other side effects may include bacterial keratitis.[1] It is a rho kinase inhibitor.[1]
Netarsudil was approved for medical use in the United States in 2017 and Europe in 2019.[1][2] In the United States a 2.5 ml bottle costs about 305 USD as of 2021.[4] It is also available as the combination netarsudil/latanoprost.[1] It is not commercially available in Europe and the United Kingdom as of 2021.[5]
Medical uses
Dosage
It is used at a dose of one drop per day.[2]
Contraindications
Netarsudil has no contraindications apart from known hypersensitivity to the drug.[2][6]
Side effects
The most common side effects are hyperaemia (increased blood flow associated with redness, in 51% of patients) in the conjunctiva, cornea verticillata (drug deposits in the cornea, in 17%), and eye pain (in 17%). All other side effects occur in fewer than 10% of people. Hypersensitivity reactions occur in fewer than 1%.[2][7]
Overdose
Overdosing netarsudil is unlikely because concentrations in the body are so low that they are generally not detectable.[7]
Interactions
No interaction studies have been done. The European label recommends to apply other eye drops at least five minutes before, and eye ointments at least five minutes after netarsudil drops.[2][6]
Pharmacology
Mechanism of action
This drug's mechanism of action is not entirely clear. It inhibits the enzyme rho kinase. This appears to increase outflow of aqueous humor through the trabecular meshwork, and also to reduce pressure in the veins of the episcleral layer. The drug also inhibits the norepinephrine transporter.[2][6]
Pharmacokinetics
After instillation into the eye, netarsudil is cleaved by esterases in the cornea to AR-13503, which is the active metabolite. Concentrations reached in the blood plasma are so low that they are generally not detectable. To judge from animal models, the drug acts for at least 24 hours. Its elimination half-life is 16 to 17 hours (in rabbits).[6]
Chemistry
The drug is used in form of a salt, netarsudil dimesilate, which is a white to light yellow crystalline powder. It is a weak acid and moderately hygroscopic, freely soluble in water and soluble in methanol.[8]
History
The FDA considers it to be a first-in-class medication.[9]
References
- 1 2 3 4 5 6 7 8 9 "Netarsudil Mesylate Monograph for Professionals". Drugs.com. Archived from the original on 3 June 2020. Retrieved 12 November 2021.
- 1 2 3 4 5 6 7 8 9 10 "Rhokiinsa EPAR". European Medicines Agency (EMA). 16 September 2019. Archived from the original on 29 December 2019. Retrieved 27 September 2020.
- ↑ "Rhopressa- netarsudil solution/ drops". DailyMed. Archived from the original on 25 March 2021. Retrieved 2 May 2021.
- ↑ "Rhopressa Prices, Coupons & Patient Assistance Programs". Drugs.com. Retrieved 12 November 2021.
- ↑ "Netarsudil". SPS - Specialist Pharmacy Service. 25 January 2016. Archived from the original on 13 November 2021. Retrieved 12 November 2021.
- 1 2 3 4 "Netarsudil Mesylate Monograph for Professionals". American Society of Health-System Pharmacists. 16 November 2020. Archived from the original on 3 June 2020. Retrieved 2 May 2021.
- 1 2 "Rhokiinsa Product Information" (PDF). European Medicines Agency (EMA). 2 December 2019. Archived (PDF) from the original on 11 June 2021. Retrieved 1 July 2021.
- ↑ "Rhokiinsa: EPAR – Public assessment report" (PDF). European Medicines Agency. 3 December 2019. Archived (PDF) from the original on 4 May 2021. Retrieved 1 July 2021.
- ↑ New Drug Therapy Approvals 2017 (PDF). U.S. Food and Drug Administration (FDA) (Report). January 2018. Archived from the original on 23 October 2020. Retrieved 16 September 2020.
External links
| External sites: |
|
|---|---|
| Identifiers: |
- "Netarsudil mesylate". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 28 October 2021. Retrieved 1 July 2021.