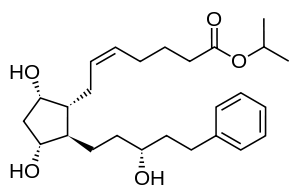
Latanoprost
 | |
 | |
| Names | |
|---|---|
| Pronunciation | la-TAN-oh-prost |
| Trade names | Xalatan, Xelpros, others |
IUPAC name
| |
| Clinical data | |
| Drug class | Prostaglandin analogue[1] |
| Main uses | Ocular hypertension, open angle glaucoma[1] |
| Side effects | Blurry vision, redness of the eye, itchiness, darkening of the iris[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | Topical eye drops |
| Onset of action | 3–4 hours |
| Duration of action | ≥ 24 hours |
| Defined daily dose | 0.1 milliliter[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a697003 |
| Legal | |
| License data |
|
| Legal status |
|
| Pharmacokinetics | |
| Metabolism | Activation by ester hydrolysis, deactivation by beta oxidation |
| Elimination half-life | 17 minutes (plasma) |
| Excretion | Mainly via kidney |
| Chemical and physical data | |
| Formula | C26H40O5 |
| Molar mass | 432.593 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Latanoprost, sold under the brand name Xalatan among others, is a medication used to treat increased pressure inside the eye.[1] This includes ocular hypertension and open angle glaucoma.[1] It is applied as eye drops to the eyes.[1] Onset of effects is usually within four hours, and they last for up to a day.[1]
Common side effects include blurry vision, redness of the eye, itchiness, and darkening of the iris.[1] Latanoprost is in the prostaglandin analogue family of medication.[1] It works by increasing the outflow of aqueous fluid from the eyes through the uveoscleral tract.[3]
Latanoprost was approved for medical use in the United States in 1996.[1] It is on the World Health Organization's List of Essential Medicines.[4] Latanoprost is available as a generic medication.[5] The wholesale cost in the developing world is about US$0.69–3.79 per 2.5 ml bottle.[6] In the United States a month of treatment costs less than US$25.[5] In 2017, it was the 81st most commonly prescribed medication in the United States with more than nine million prescriptions.[7][8]
Medical uses
Open-angle glaucoma
In people with ocular hypertension (intraocular pressure (IOP) ≥21 mm Hg) including open-angle glaucoma, treatment with latanoprost reduced IOP levels by 22 to 39% over 1 to 12 months’ treatment. Latanoprost was more effective than timolol 0.5% twice daily in 3 of 4 large (n = 163 to 267) randomised, double-blind trials. Latanoprost demonstrated a stable long-term IOP-lowering effect in 1- or 2-year continuations of these trials, with no sign of diminishing effect during prolonged treatment.[9]
Meta-analysis suggests that latanoprost is more effective than timolol in lowering intraocular pressure (IOP). However, it often causes iris pigmentation. While current evidence suggests that this pigmentation is benign, careful lifetime evaluation of patients is still justified.[10]
Closed-angle glaucoma
Patients who had elevated IOP despite iridotomy and/or iridectomy (including patients of Asian descent), latanoprost was significantly more effective than timolol in two double-blind, monotherapy trials (8.2 and 8.8 mm Hg vs 5.2 and 5.7 mm Hg for latanoprost vs timolol at 12 and 2 weeks, respectively).[11]
Dosage
The defined daily dose is 0.1 ml (via eye/opthalmic drops)[2]
Side effects
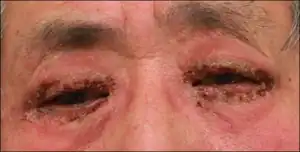
Listed from most to least common:[12][13]
- > 5–15%: blurred vision, burning and stinging, conjunctival hyperemia, foreign body sensation, itching, increased (brown) pigmentation of the iris (causing heterochromia), punctate epithelial keratopathy
- 4%: cold or upper respiratory tract infections, flu-like syndrome
- 1–4%: dry eyes, excessive tearing, eye pain, lid crusting, lid edema, lid erythema (hyperemia), lid pain, photophobia
- 1–2%: chest pain, allergic skin reactions, arthralgia, back pain, myalgia
- < 1 % (only severe or life-threatening effects): asthma, herpes keratitis, iritis, keratitis, retinal artery embolus, retinal detachment, toxic epidermal necrolysis, uveitis, vitreous hemorrhage from diabetic retinopathy
- A single case report links latanoprost use to the progression of keratoconus.[14]
Research suggests that wiping the eye with an absorbent pad after the administration of eye drops can result in shorter eyelashes and a lesser chance of hyperpigmentation in the eyelid, compared to not wiping off excess fluid.[15]
Pregnancy
Use in pregnant women is limited due to high incidence of abortion shown in animal experiments. Because of this, latanoprost is classified as risk factor C (adverse events were observed in animal reproduction studies at maternally toxic doses) according to United States Food and Drug Administration's use-in-pregnancy ratings.[16] Drug excretion in breast milk is unknown.[3]
Interactions
Interactions are similar to other prostaglandin analogs. Paradoxically, the concomitant use of latanoprost and bimatoprost or other prostaglandins may result in increased intraocular pressure. Non-steroidal anti-inflammatory drugs (NSAIDs) can reduce or increase the effect of latanoprost.[12][13]
Pharmacology
Mechanism of action
Like tafluprost and travoprost, latanoprost is an ester prodrug that is activated to the free acid in the cornea. Also like the related drugs, latanoprost acid is an analog of prostaglandin F2α that acts as a selective agonist at the prostaglandin F receptor. Prostaglandins increase the sclera's permeability to aqueous fluid. So, an increase in prostaglandin activity increases outflow of aqueous fluid thus lowering intraocular pressure.[12][13]
Pharmacokinetics
Latanoprost is absorbed well through the cornea and completely hydrolysed to the active latanoprost acid. Highest concentrations of the acid in the aqueous humour are reached two hours after application, lowering of intraocular pressure starts after 3 to 4 hours, the highest effect is found after 8 to 12 hours, and its action lasts at least 24 hours. When latanoprost acid reaches the circulation, it is quickly metabolised in the liver by beta oxidation to 1,2-dinor- and 1,2,3,4-tetranor-latanoprost acid; blood plasma half life is only 17 minutes. The metabolites are mainly excreted via the kidney.[12][13]
The activation and deactivation pathway is analogous to the one of tafluprost; see Tafluprost#Pharmacokinetics for chemical formulae.
Chemistry
Stability
Latanoprost exhibits thermal and solar instability. The concentration of latanoprost stored at 50 °C will decrease by 10% every 8.25 days. When stored at 70 °C the concentration will decrease by 10% every 1.32 days. Ultraviolet light, for example in sunlight, causes rapid degradation of latanoprost.[17]
Society and culture
The brand Xalatan is manufactured by Pfizer.
 Latanoprost eye drops, marketed by Pfizer
Latanoprost eye drops, marketed by Pfizer Latanoprost in Japanese-language packaging
Latanoprost in Japanese-language packaging
Cost
The wholesale cost in the developing world is about US$0.69–3.79 per 2.5 ml bottle.[6] In the United States a month of treatment costs less than US$25.[5] In 2017, it was the 81st most commonly prescribed medication in the United States with more than nine million prescriptions.[7][8]
.svg.png.webp) Latanoprost costs (US)
Latanoprost costs (US).svg.png.webp) Latanoprost prescriptions (US)
Latanoprost prescriptions (US)
Cosmetic use
- Lengthening and thickening of the eyelashes (used, like bimatoprost, in the cosmetic industry as eyelash growth enhancers).[18]
- There is one small study that found benefit in androgenic alopecia.[19]
See also
References
- 1 2 3 4 5 6 7 8 9 10 "Latanoprost". The American Society of Health-System Pharmacists. Archived from the original on 28 December 2016. Retrieved 8 December 2016.
- 1 2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 23 January 2021. Retrieved 16 September 2020.
- 1 2 Patel SS, Spencer CM (1996). "Latanoprost. A review of its pharmacological properties, clinical efficacy and tolerability in the management of primary open-angle glaucoma and ocular hypertension". Drugs Aging. 9 (5): 363–378. doi:10.2165/00002512-199609050-00007. PMID 8922563.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- 1 2 3 Hamilton, Richart (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 413. ISBN 9781284057560.
- 1 2 "Latanoprost". International Drug Price Indicator Guide. Retrieved 8 December 2016.
- 1 2 "The Top 300 of 2020". ClinCalc. Archived from the original on 12 February 2021. Retrieved 11 April 2020.
- 1 2 "Latanoprost - Drug Usage Statistics". ClinCalc. Archived from the original on 8 July 2020. Retrieved 11 April 2020.
- ↑ Perry CM, McGavin JK, Culy CR, Ibbotson T (2003). "Latanoprost. An Update of its Use in Glaucoma and Ocular Hypertension". Drugs & Aging. 20 (8): 597–630. doi:10.2165/00002512-200320080-00005. PMID 12795627.
- ↑ Zhang WY, Wan Po AL, Dua HS, Azuara-Blanco A (2001). "Meta-analysis of randomised controlled trials comparing latanoprost with timolol in the treatment of patients with open angle glaucoma or ocular hypertension". British Journal of Ophthalmology. 85 (8): 983–990. doi:10.1136/bjo.85.8.983. PMC 1724079. PMID 11466259.
- ↑ Aung T; Wong HT; Yip CC; et al. (2000). "Comparison of the intraocular pressure-lowering effect of latanoprost and timolol in patients with chronic angle closure glaucoma: a preliminary study". Ophthalmology. 107 (6): 1178–83. doi:10.1016/s0161-6420(00)00073-7. PMID 10857840.
- 1 2 3 4 Latanoprost Professional Drug Facts.
- 1 2 3 4 Haberfeld, H, ed. (2015). Austria-Codex (in German). Vienna: Österreichischer Apothekerverlag.
{{cite book}}: CS1 maint: unrecognized language (link) - ↑ Amano S, Nakai Y, Ko A, Inoue K, Wakakura M (2008). "A case of keratoconus progression associated with the use of topical latanoprost". Japanese Journal of Ophthalmology. 52 (4): 334–6. doi:10.1007/s10384-008-0554-6. PMID 18773275.
- ↑ Xu L, Wang X, Wu M (2017). "Topical medication instillation techniques for glaucoma". Cochrane Database Syst Rev. 2: CD010520. doi:10.1002/14651858.CD010520.pub2. PMC 5419432. PMID 28218404.
- ↑ De Santis, M; Lucchese, A; Carducci, B; Cavaliere, A. F.; De Santis, L; Merola, A; Straface, G; Caruso, A (2004). "Latanoprost exposure in pregnancy". American Journal of Ophthalmology. 138 (2): 305–6. doi:10.1016/j.ajo.2004.03.002. PMID 15289149.
- ↑ Morgan PV, Proniuk S, Blanchard J, Noecker RJ (2001). "Effect of temperature and light on the stability of latanoprost and its clinical relevance". Journal of Glaucoma. 10 (5): 401–405. doi:10.1097/00061198-200110000-00007. PMID 11711838.
- ↑ Johnstone, Murray A.; Albert, Daniel M. (1 August 2002). "Prostaglandin-Induced Hair Growth". Survey of Ophthalmology. 47: S185–S202. doi:10.1016/S0039-6257(02)00307-7. ISSN 0039-6257. PMID 12204716. Archived from the original on 28 August 2021. Retrieved 1 October 2019.
- ↑ Gupta, AK; Mays, RR; Versteeg, SG; Shear, NH; Piguet, V; Piraccini, BM (March 2019). "Efficacy of Off-Label Topical Treatments for the Management of Androgenetic Alopecia: A Review". Clinical Drug Investigation. 39 (3): 233–239. doi:10.1007/s40261-018-00743-8. PMID 30652260.
External links
| External sites: |
|
|---|---|
| Identifiers: |