Selenium disulfide
| Names | |
|---|---|
| Trade names | Selsun Blue,[1] Selseb, others |
| Other names | Selenium sulfide |
IUPAC name
| |
| Clinical data | |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | Topical |
| Defined daily dose | not established[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| Legal status |
|
| Chemical and physical data | |
| Molar mass | 143.09 |
| Density | 3 g/cm3 |
| Melting point | 111 °C (232 °F) |
| Boiling point | 118 to 119 °C (244 to 246 °F) (decomposes) |
| Solubility in water | negligible mg/mL (20 °C) |
Selenium disulfide, also known as selenium sulfide, is a chemical compound and medication used to treat pityriasis versicolor, seborrhoeic dermatitis, and dandruff.[3] It is applied to the affected area as a lotion or shampoo.[4] Dandruff frequently returns if treatment is stopped.[5]
Side effects include hair loss, irritation of the skin, weakness, and feeling tired.[3] Use is not recommended in children less than 2–5 years old.[3][5] Use in pregnancy or breastfeeding has not been studied.[6] Selenium disulfide is an inorganic compound with the chemical formula SeS2.[7]
Selenium disulfide was approved for medical use in the United States at least as early as 1951.[5] It is on the World Health Organization's List of Essential Medicines.[8] Selenium disulfide is available as a generic medication and over the counter.[4] In the United States a month of treatment costs less than 25 USD.[4] In the United Kingdom 100 ml of 2.5% shampoo costs the NHS about 1.96 pounds.[9]
Medical uses
Selenium disulfide is sold as an antifungal agent in shampoos for the treatment of dandruff and seborrheic dermatitis associated in the scalp with fungi of genus Malassezia.[10][11][12] In the United States, a 1% strength is available over-the-counter, and a 2.5% strength is also available with a prescription. In Canada, the 2.5% strength is available over-the-counter. At the 2.5% strength, selenium disulfide is also used on the body to treat Tinea versicolor, a type of fungal skin infection caused by a different species of Malassezia. It has been suggested to be effective as a treatment for hyperkeratosis.[13]
Dosage
The defined daily dose is not established.[2]
Side effects
Selenium disulfide can cause discoloration of the hair and alter the color of hair dyes. It may also discolor metallic jewellery.
Chemical composition
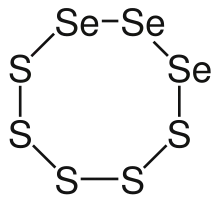
Selenium disulfide has a composition that approximates to SeS2 and is sometimes called selenium sulfide. However, as used in proprietary formulations, it is not a pure chemical compound but a mixture where the overall Se:S ratio is 1:2. The compounds are Se–S rings containing a variable number of S and Se atoms, SenS8−n.[14]
Many selenium sulfides are known as indicated by 77Se-NMR spectroscopy.[15]
History
Selenium monosulfide, along with elemental selenium and sulfur, has been used in medicinal preparations in the past,[16] causing confusion and contradiction[17] as to exactly what form selenium is in any given topical preparation.
Society and culture
In the film Evolution selenium was mentioned as an active ingredient of Head & Shoulders. A group of academics, therefore, tried to use this brand of shampoo to stop an alien invasion after discovering that the alien life form was sensitive to selenium. In fact, the modern shampoo from this brand no longer contains selenium sulfide and instead zinc pyrithione is used as a dandruff-reducing component.[18]
See also
- Zinc pyrithione, an antimicrobial agent used in many off the shelf shampoos
- Selsun Blue, a shampoo with selenium disulfide as its active ingredient
- Ketoconazole, another antifungal agent used in medicated shampoos
References
- ↑ Gupta, Aditya K.; Mays, Rachel R.; Folley, Kelly A. (2019). "42. Topical antifungal agents". In Wolverton, Stephen E.; Wu, Jashin J. (eds.). Comprehensive Dermatologic Drug Therapy (4th ed.). Elsevier. p. 488. ISBN 978-0-323-61211-1. Archived from the original on 2021-10-09. Retrieved 2021-10-07.
- 1 2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 20 September 2020. Retrieved 13 September 2020.
- 1 2 3 World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. p. 297. hdl:10665/44053. ISBN 9789241547659.
- 1 2 3 Hamilton, Richart (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 194. ISBN 9781284057560.
- 1 2 3 "Selenium Sulfide". The American Society of Health-System Pharmacists. Archived from the original on 18 January 2017. Retrieved 8 January 2017.
- ↑ "Selenium sulfide topical Use During Pregnancy | Drugs.com". www.drugs.com. Archived from the original on 16 January 2017. Retrieved 13 January 2017.
- ↑ Mitchell, Stephen C. (2003). Biological Interactions Of Sulfur Compounds. CRC Press. p. 174. ISBN 9780203362525. Archived from the original on 2017-01-16.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ British national formulary : BNF 69 (69 ed.). British Medical Association. 2015. p. 829. ISBN 9780857111562.
- ↑ Selenium(IV) sulfide - pharmacy codes search engine Archived 2008-04-01 at the Wayback Machine
- ↑ Chemicals of Selenium .Se Archived 2008-04-03 at the Wayback Machine
- ↑ Accessed Dec. 24, 2007 Archived 2008-12-26 at the Wayback Machine
- ↑ Cohen PR, Anderson CA (December 2018). "Topical Selenium Sulfide for the Treatment of Hyperkeratosis". Dermatology and therapy. 8 (4): 639–46. doi:10.1007/s13555-018-0259-9. PMC 6261123. PMID 30203232.
- ↑ Cyclic selenium sulfides R. Steudel, R. Laitinen, Topics in Current Chemistry, (1982), 102, 177-197
- ↑ Pekonen, Pentti.; Hiltunen, Yrjō; Laitinen, Risto S.; Pakkanen, Tapani A. (1991). "Chalcogen ring interconversion pathways. 77Se NMR spectroscopic study of the decomposition of 1,2,3,4,5-Se5S2 to 1,2,3,4,5,6-Se6S2 and 1,2,3,4-Se4S2". Inorganic Chemistry. 30 (19): 3679. doi:10.1021/ic00019a022.
- ↑ "Definition: selenium sulfide from Online Medical Dictionary".
- ↑ "DrugBank: DB00971 (Selenium Sulfide)". Archived from the original on 2007-04-27.
- ↑ "Evolution (2001) - IMDb". Archived from the original on 8 November 2020. Retrieved 19 May 2020.
Further reading
- Danby, FW; Maddin, WS; Margesson, LJ; Rosenthal, D (December 1993). "A randomized, double-blind, placebo-controlled trial of ketoconazole 2% shampoo versus selenium sulfide 2.5% shampoo in the treatment of moderate to severe dandruff". Journal of the American Academy of Dermatology. 29 (6): 1008–12. doi:10.1016/0190-9622(93)70282-x. PMID 8245236. Archived from the original on 2013-04-14. Retrieved 2012-05-31.
- Grover, R. W. (1956). "Diffuse Hair Loss Associated with Selenium (Selsun) Sulfide Shampoo". JAMA: The Journal of the American Medical Association. 160 (16): 1397. doi:10.1001/jama.1956.02960510023006.
- Givens, T. G.; Murray, M. M.; Baker, R. C. (1995). "Comparison of 1% and 2.5% Selenium Sulfide in the Treatment of Tinea Capitis". Archives of Pediatrics and Adolescent Medicine. 149 (7): 808–11. doi:10.1001/archpedi.1995.02170200098016. PMID 7795774.
- Ransone, James W.; Scott, Norman M.; Knoblock, Edward C. (1961). "Selenium Sulfide Intoxication". New England Journal of Medicine. 264 (8): 384. doi:10.1056/NEJM196102232640806. PMID 13739506.
- Laitinen, Risto S.; Pakkanen, Tapani A. (1987). "77Se NMR spectroscopic characterization of selenium sulfide ring molecules SenS8−n". Inorganic Chemistry. 26 (16): 2598. doi:10.1021/ic00263a010.
External links
| Identifiers: |
|---|
- "Selenium Disulfide". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2021-08-29. Retrieved 2020-05-17.