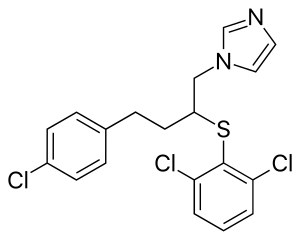
Butoconazole
 | |
| Names | |
|---|---|
| Trade names | Gynazole-1, Femstat-3, others |
IUPAC name
| |
| Clinical data | |
| Drug class | Antifungal[1] |
| Main uses | Vaginal yeast infections[1] |
| Side effects | Burning, itchiness, abdominal pain[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | Vaginal cream |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682012 |
| Legal | |
| License data |
|
| Legal status |
|
| Chemical and physical data | |
| Formula | C19H17Cl3N2S |
| Molar mass | 411.77 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Butoconazole, sold under the brand name Gynazole-1 among others, is antifungal used to treat vaginal yeast infections.[1] It is used inside the vagina.[1]
Common side effects include burning, itchiness, and abdominal pain.[1] Its use may weaken condoms in the 3 days following application.[1] It may be used in pregnancy.[1] It is an imidazole.[1]
Butoconazole was first made in 1978 and approved for medical use in the United States in 1995.[2][1] It is available over the counter.[1] In the United States a dose costs about 105 USD as of 2022.[3]
Medical uses
Dosage
It is generally used once a day for one to three days.[1] For complicated cases up to 14 days may be used.[1]
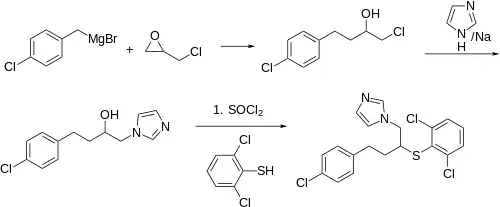
Synthesis
Reaction of epichlorohydrin with 4-Chlorobenzyl magnesium bromide leads to 1-chloro-4-(4-chlorophenyl)butan-2-ol (3). Displacement with sodium imidazole, conversion of the secondary alcohol to the chloride (SOCl2), and displacement with 2,6-dichlorobenzenethiol concludes the synthesis of the antifungal butoconazole.
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 "Butoconazole Monograph for Professionals". Drugs.com. Archived from the original on 3 January 2022. Retrieved 12 January 2022.
- ↑ Grayson, M. Lindsay; Crowe, Suzanne M.; McCarthy, James S.; Mills, John; Mouton, Johan W.; Norrby, S. Ragnar; Paterson, David L.; Pfaller, Michael A. (29 October 2010). Kucers' The Use of Antibiotics Sixth Edition: A Clinical Review of Antibacterial, Antifungal and Antiviral Drugs. CRC Press. p. 1893. ISBN 978-1-4441-4752-0. Archived from the original on 12 January 2022. Retrieved 12 January 2022.
- ↑ "Gynazole-1 Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 29 October 2016. Retrieved 12 January 2022.
- ↑ Walker KA, Braemer AC, Hitt S, Jones RE, Matthews TR (August 1978). "1-[4-(4-Chlorophenyl)-2-(2,6-dichlorophenylthio)-n-butyl]-1H-imidazole nitrate, a new potent antifungal agent". Journal of Medicinal Chemistry. 21 (8): 840–3. doi:10.1021/jm00206a028. PMID 357722.
- ↑ US 4078071, Walker KA, "Derivatives of substituted N-alkyl imidazoles", issued 7 March 1978, assigned to Syntex
External links
| External sites: |
|
|---|---|
| Identifiers: |