Voriconazole
| Names | |
|---|---|
| Pronunciation | /vɒrɪˈkɒnəzoʊl/ vorr-i-KON-ə-zohl |
| Trade names | Vfend, others |
IUPAC name
| |
| Clinical data | |
| Main uses | Fungal infections[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | IV, by mouth (tablet, suspension) |
| Defined daily dose | 0.4 mg[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a605022 |
| Legal | |
| License data |
|
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | 96% (oral) |
| Protein binding | 58% |
| Metabolism | Liver: CYP2C19 (significant involvement), also CYP2C9, CYP3A4 |
| Metabolites | Voriconazole N-oxide (major; minimal antifungal activity) |
| Elimination half-life | Dose-dependent |
| Excretion | Urine (80–83%)[3] |
| Chemical and physical data | |
| Formula | C16H14F3N5O |
| Molar mass | 349.317 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Voriconazole, sold under the brand name Vfend among others, is an antifungal medication used to treat a number of fungal infections.[1] This includes aspergillosis, candidiasis, coccidioidomycosis, histoplasmosis, penicilliosis, and infections by Scedosporium or Fusarium.[1] It can be taken by mouth or used by injection into a vein.[1]
Common side effects include vision problems, nausea, abdominal pain, rash, headache, and seeing or hearing things that are not present.[1] Use during pregnancy may result in harm to the baby.[1] It is in the triazole family of medications.[1] It works by affecting fungal metabolism and fungal cell membranes.[1]
Voriconazole was patented in 1990 and approved for medical use in the United States in 2002.[4][5] It is on the World Health Organization's List of Essential Medicines.[6] The wholesale cost in the United States, as of 2019, is about US$9 per day.[7]
Medical uses
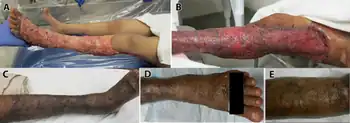
Voriconazole is used to treat invasive aspergillosis and candidiasis and fungal infections caused by Scedosporium and Fusarium species, which may occur in immunocompromised patients, including people undergoing allogeneic bone marrow transplant (BMT), who have hematologic cancers or who undergo organ transplants.[9][10][11][12]
It is also used to prevent fungal infection in people as they undergo BMT.[11][9]
It is also the recommended treatment for the CNS fungal infections transmitted by epidural injection of contaminated steroids.[13]
It can be taken by mouth or given by intravenous infusion.[9]
Dosage
The defined daily dose is 0.4 mg by mouth or by injection.[2]
Side effects
The labels carry several warnings of the risk of injection site reactions, hypersensitivity reactions; kidney, liver, and pancreas damage; trouble with vision; and adverse effects in skin including damage due to phototoxicity, squamous cell skin cancer, and Stevens–Johnson syndrome; in long-term use there is a warning of the risk of bone fluorosis and periostitis.[3][9]
Additionally, very common adverse effects, occurring in more than 10% of people, include peripheral edema, headaches, trouble breathing, diarrhea, vomiting, abdominal pain, nausea, rashes, and fever.[9]
Common side effects, occurring in between 1 and 10% of people, include sinus infections, low numbers of white and red blood cells (agranulocytosis, pancytopenia, thrombocytopenia, leukopenia, and anemia), low blood sugar, reduced amount of potassium and sodium, depression, hallucinations, anxiety, insomnia, agitation, confusion, convulsions, fainting, tremor, weakness, tingling, sleepiness, dizziness, bleeding retina, irregular heart beats, slow or fast heart beats, low blood pressure, inflamed veins, acute respiratory distress syndrome, pulmonary edema, inflamed lips, swollen face, stomach upset, constipation, gingivitis, jaundice, hair loss, flaky skin, itchiness, red skin, back pain, chest pain, and chills.[9]
People who have hereditary intolerance for galactose, Lapp lactase deficiency, or glucose-galactose malabsorption should not take it. It should be used with caution in people with arrhythmias or long QT.[3]
No dose adjustment is necessary for renal impairment or advanced age, but children seem to clear voriconazole faster than adults and drug levels may need monitoring.[14]
Pregnancy and breastfeeding
Use during pregnancy may harm the baby; thus pregnant women should not take it and women taking it should not become pregnant.[3]
Interactions
Being metabolized by hepatic cytochrome P450, voriconazole interacts with many drugs.[3][9] Voriconazole should not be used in conjunction with many drugs (including sirolimus, rifampicin, rifabutin, carbamazepine, quinidine and ergot alkaloids) and dose adjustments and/or monitoring should be done when coadministered with others (including fluconazole, warfarin, ciclosporin, tacrolimus, omeprazole, and phenytoin). Voriconazole may be safely administered with cimetidine, ranitidine, indinavir, macrolide antibiotics, mycophenolate, digoxin and prednisolone.[3]
Pharmacology
Pharmacokinetics
Voriconazole is well absorbed when taken by mouth with a bioavailability of 96%, allowing people to be switched between intravenous and by mouth formulations.
History
Pfizer brought the drug to market as Vfend. A generic version of the tablet form of voriconazole was introduced in the US in 2011 after Pfizer and Mylan settled litigation under the Hatch-Waxman Act; a generic version of the injectable form was introduced in 2012. In Europe patent protection expired in 2011 and pediatric administrative exclusivity expired in Europe in 2016.[15]
Society and culture
Brand names
As of July 2017 the drug was marketed under the following names worldwide: Cantex, Pinup, Vedilozin, Vfend, Vodask, Volric, Voramol, Voriconazol, Voriconazole, Voriconazolum, Voricostad, Vorikonazol, Voritek, Voriz, Vornal, and Vosicaz.[16]
References
- 1 2 3 4 5 6 7 8 "Voriconazole". The American Society of Health-System Pharmacists. Archived from the original on 10 December 2017. Retrieved 8 December 2017.
- 1 2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 21 October 2020. Retrieved 11 September 2020.
- 1 2 3 4 5 6 "US voriconazole label" (PDF). FDA. June 2017. Archived (PDF) from the original on 29 March 2021. Retrieved 30 July 2017. For label updates see FDA index page for NDA 021630 Archived 2017-06-29 at the Wayback Machine
- ↑ Kendig, Edwin L.; Wilmott, Robert W.; Chernick, Victor (2012). Kendig and Chernick's Disorders of the Respiratory Tract in Children. Elsevier Health Sciences. p. 539. ISBN 978-1437719840. Archived from the original on 2017-12-10. Retrieved 2017-12-10.
- ↑ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 503. ISBN 9783527607495. Archived from the original on 2020-08-02. Retrieved 2019-03-02.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ "NADAC as of 2019-08-21". Centers for Medicare and Medicaid Services. Retrieved 2019-09-23.
{{cite web}}: CS1 maint: url-status (link) - ↑ El-Herte, Rima I.; Schouweiler, Katie E.; Farah, Ronda S.; Arbulu, Ricardo; Diekema, Daniel; Wanat, Karolyn A.; Ford, Bradley A. (16 October 2014). "Phaeoacremonium parasiticum phaeohyphomycosis in a patient with systemic lupus erythematosus treated successfully with surgical debridement and voriconazole: A case report and review of the literature". IDCases. 1 (4): 84–88. doi:10.1016/j.idcr.2014.10.004. ISSN 2214-2509. Archived from the original on 1 February 2022. Retrieved 1 February 2022.
- 1 2 3 4 5 6 7 "Vfend tablet and powder". UK Electronic Medicines Compendium. January 2017. Archived from the original on 31 July 2017. Retrieved 30 July 2017.
- ↑ Patterson, TF; Thompson GR, 3rd; Denning, DW; Fishman, JA; Hadley, S; Herbrecht, R; Kontoyiannis, DP; Marr, KA; Morrison, VA; Nguyen, MH; Segal, BH; Steinbach, WJ; Stevens, DA; Walsh, TJ; Wingard, JR; Young, JA; Bennett, JE (15 August 2016). "Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America". Clinical Infectious Diseases. 63 (4): e1–e60. doi:10.1093/cid/ciw326. PMC 4967602. PMID 27365388.
- 1 2 Omrani, AS; Almaghrabi, RS (13 June 2017). "Complications of hematopoietic stem transplantation: Fungal infections". Hematology/Oncology and Stem Cell Therapy. 10 (4): 239–244. doi:10.1016/j.hemonc.2017.05.013. PMID 28636889.
- ↑ Herbrecht, R; Denning, DW; Patterson, TF; Bennett, JE; Greene, RE; Oestmann, JW; Kern, WV; Marr, KA; Ribaud, P; Lortholary, O; Sylvester, R; Rubin, RH; Wingard, JR; Stark, P; Durand, C; Caillot, D; Thiel, E; Chandrasekar, PH; Hodges, MR; Schlamm, HT; Troke, PF; de Pauw, B (8 August 2002). "Voriconazole versus Amphotericin B for Primary Therapy of Invasive Aspergillosis" (PDF). The New England Journal of Medicine. 347 (6): 408–15. doi:10.1056/NEJMoa020191. hdl:2066/185528. PMID 12167683. Archived from the original on 27 August 2021. Retrieved 24 September 2019.
- ↑ "Interim Treatment Guidance for Central Nervous System and Parameningeal Infections Associated with Injection of Contaminated Steroid Products". Centers for Disease Control and Prevention. Archived from the original on 18 November 2016. Retrieved 6 November 2016.
- ↑ Smith, J; Safdar, N; Knasinski, V; Simmons, W; Bhavnani, SM; Ambrose, PG; Andes, D (April 2006). "Voriconazole Therapeutic Drug Monitoring". Antimicrobial Agents and Chemotherapy. 50 (4): 1570–2. doi:10.1128/AAC.50.4.1570-1572.2006. PMC 1426935. PMID 16569888.
- ↑ "Vfend loses its paediatric protection" (PDF). IMS Health Generics Bulletin. 22 July 2016. Archived from the original on 2 October 2021. Retrieved 30 July 2017.
- ↑ "Voriconazole international brand names". Drugs.com. Archived from the original on 31 July 2017. Retrieved 30 July 2017.
Further reading
- Dean L (December 2019). "Voriconazole Therapy and CYP2C19 Genotype". In Pratt VM, McLeod HL, Rubinstein WS, et al. (eds.). Medical Genetics Summaries. National Center for Biotechnology Information (NCBI). PMID 31886997. Archived from the original on 2020-10-26. Retrieved 2020-04-15.
External links
| External sites: |
|
|---|---|
| Identifiers: |