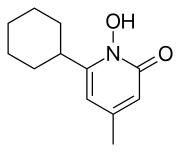
Ciclopirox
 | |
| Names | |
|---|---|
| Trade names | Loprox, Penlac, others |
IUPAC name
| |
| Clinical data | |
| Drug class | Antifungal[1] |
| Main uses | Ringworm, tinea versicolor, candidiasis of the skin, seborrheic dermatitis, fungal infection of the nail[1] |
| Side effects | Mild redness, burning[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | Topical (applied as a nail lacquer, skin cream or shampoo) |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a604021 |
| Legal | |
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | <5% with prolonged use |
| Protein binding | 94 to 97% |
| Elimination half-life | 1.7 hours |
| Chemical and physical data | |
| Formula | C12H17NO2 |
| Molar mass | 207.269 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Ciclopirox (CPX) is an antifungal used to treat ringworm, tinea versicolor, candidiasis of the skin, seborrheic dermatitis, and fungal infection of the nail.[1] It is applied as a gel, cream, or nail polish.[2] Use on toenails is only recommended when less than 40% of the nail is involved.[3]
It is generally well tolerated.[2] Common side effects include mild redness and burning.[1] Other side effects may include allergic reactions.[2] While there is no evidence of harm in pregnancy, such use has not been well studied.[4] Its mechanism of action differs from other antifungals.[2]
Ciclopirox was approved for medical use in the United States in 1982.[1] It is available as a generic medication.[5] In the United States a bottle of 6.6 ml of the nail polish costs about 16 USD as of 2022.[5]
Medical uses
Ciclopirox is indicated for the treatment of tinea pedis and tinea corporis due to Trichophyton rubrum, Trichophyton mentagrophytes and Epidermophyton floccosum, as well as seborrheic dermatitis. It is not to be used in the eyes or vagina, and nursing women should consult their doctors before use, since it is not known whether ciclopirox passes into human milk. A burning sensation may be felt when first applying ciclopirox on the skin.[6]
Nail infections
In addition to other formulations, ciclopirox is used in lacquers for topical treatment of onychomycosis (fungal infections of the nails). A 2009 review concluded that topical ciclopirox had poor cure rates and that amorolfine might be more effective.
"Combining data from 2 trials of ciclopiroxolamine versus placebo found treatments failure rates of 61% and 64% for ciclopiroxolamine. These outcomes followed long treatment times (48 weeks) and this makes ciclopiroxolamine a poor choice for nail infections. Better results were observed with the use of amorolfine lacquer; 6% treatment failure rates were found after 1 month of treatment but these data were collected on a very small sample of people and these high rates of success might be unreliable."[7]
Pharmacology
In contrast to the azoles and other antimycotic drugs, the mechanism of action of ciclopirox is poorly understood.[8] However, loss of function of certain catalase and peroxidase enzymes has been implicated as the mechanism of action, as well as various other components of cellular metabolism. In a study conducted to further elucidate ciclopirox's mechanism, several Saccharomyces cerevisiae mutants were screened and tested. Results from interpretation of the effects of both the drug treatment and mutation suggested that ciclopirox may exert its effect by disrupting DNA repair, cell division signals and structures (mitotic spindles) as well as some elements of intracellular transport.[9]
It is currently being investigated as an alternative treatment to ketoconazole for seborrhoeic dermatitis as it suppresses growth of the yeast Malassezia furfur. Initial results show similar efficacy to ketoconazole with a relative increase in subjective symptom relief due to its inherent anti-inflammatory properties.[10]
Chemistry
Ciclopirox is a considered a hydroxypyrimidine (sic) antifungal agent. Structurally, ciclopirox is the N-oxide of a 2-hydroxypyridine derivative and therefore ought to be termed a hydroxypyridine antifungal agent. Additionally, the structure as drawn above is the lactam tautomer and indicates the molecule being an N-Hydroxy-2-pyridone. Hence the classification of ciclopirox as a 2-pyridone antifungal agent.
Ciclopirox is used clinically as ciclopirox olamine, the olamine salt of ciclopirox.
Society and culture
It is sold under many brand names worldwide.[11]
Cost
The topical cream 0.77% is about $25 (USD) for 15 grams in the U.S.[12]
.svg.png.webp) Ciclopirox costs (US)
Ciclopirox costs (US).svg.png.webp) Ciclopirox prescriptions (US)
Ciclopirox prescriptions (US)
References
- 1 2 3 4 5 6 "Ciclopirox Monograph for Professionals". Drugs.com. Archived from the original on 25 January 2021. Retrieved 4 January 2022.
- 1 2 3 4 Gupta, Aditya K.; Mays, Rachel R.; Folley, Kelly A. (2019). "42. Topical antifungal agents". In Wolverton, Stephen E.; Wu, Jashin J. (eds.). Comprehensive Dermatologic Drug Therapy (4th ed.). Elsevier. p. 487. ISBN 978-0-323-61211-1. Archived from the original on 2021-10-09. Retrieved 2021-10-07.
- ↑ Ton, Joey (3 September 2019). "#242 Putting the FUN in Fungi: Toenail onychomycosis treatments". CFPCLearn. Archived from the original on 28 March 2023. Retrieved 15 June 2023.
- ↑ "Ciclopirox topical Use During Pregnancy". Drugs.com. Archived from the original on 21 October 2020. Retrieved 4 January 2022.
- 1 2 "Ciclopirox Prices and Ciclopirox Coupons - GoodRx". GoodRx. Archived from the original on 1 November 2016. Retrieved 4 January 2022.
- ↑ "Ciclopirox Olamine Antifungal Shampoo". Okdermo. Archived from the original on 2020-08-10. Retrieved 2019-08-06.
- ↑ Crawford F (1996). "Topical treatments for fungal infections of the skin and nails of the foot". Reviews. 319 (7202): 79–82. doi:10.1002/14651858.CD001434.pub2. PMC 28154. PMID 10398626.
- ↑ Niewerth M, Kunze D, Seibold M, Schaller M, Korting HC, Hube B (June 2003). "Ciclopirox Olamine Treatment Affects the Expression Pattern of Candida albicans Genes Encoding Virulence Factors, Iron Metabolism Proteins, and Drug Resistance Factors". Antimicrobial Agents and Chemotherapy. 47 (6): 1805–1817. doi:10.1128/AAC.47.6.1805-1817.2003. PMC 155814. PMID 12760852.
- ↑ Leem SH, Park JE, Kim IS, Chae JY, Sugino A, Sunwoo Y (2003). "The possible mechanism of action of ciclopirox olamine in the yeast Saccharomyces cerevisiae". Mol. Cells. 15 (1): 55–61. PMID 12661761. Archived from the original on 2009-06-20. Retrieved 2021-03-23.
- ↑ Ratnavel RC, Squire RA, Boorman GC (2007). "Clinical efficacies of shampoos containing ciclopirox olamine (1.5%) and ketoconazole (2.0%) in the treatment of seborrhoeic dermatitis". J Dermatolog Treat. 18 (2): 88–96. doi:10.1080/16537150601092944. PMID 17520465. S2CID 34852507.
- ↑ Drugs.com International brand names for ciclopirox Archived 2019-08-04 at the Wayback Machine Page accessed January 201, 2016
- ↑ "Ciclopirox topical Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 19 January 2021. Retrieved 26 March 2021.
External links
| Identifiers: |
|---|