Acetarsol
 | |
| Names | |
|---|---|
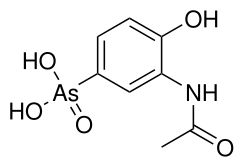
| Preferred IUPAC name
(3-Acetamido-4-hydroxyphenyl)arsonic acid | |
| Other names
Acetarsone | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.349 |
| EC Number |
|
| KEGG | |
| MeSH | Acetarsol |
PubChem CID |
|
| UNII | |
| UN number | 3465 |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C8H10AsNO5 |
| Molar mass | 275.0903 g mol−1 |
| Pharmacology | |
| A07AX02 (WHO) G01AB01 (WHO), P01CD02 (WHO), P51AD05 (WHO) | |
| Hazards | |
| GHS labelling: | |
Pictograms |
  |
Signal word |
Danger |
Hazard statements |
H301, H331, H410 |
Precautionary statements |
P261, P273, P301+P310, P311, P501 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Acetarsol (or acetarsone[1]) is an anti-infective drug.[2]
It was first discovered in 1921 at Pasteur Institute by Ernest Fourneau,[3] and sold under the brand name Stovarsol.[4][5]
It has been given in the form of suppositories.[6]
Acetarsol can be used to make arsthinol.
It has been cancelled and withdrawn from the market since August 12th, 1997. [3]
Medical Uses
Acetarsol has been used for the treatment of different diseases such as syphillus, amoebiasis, yaws, trypanosomiasisiasis and malaria. Acetarsol was used for the treatment of Trichomonas Vaginalis and Candida Albicans. In the oral form, Acetarsol can be used for the treatment of intestinal amoebiasis. As a suppository, Acetarsol was researched to be used for the treatment of proctitis.[3]
Mechanism of Action
Although the mechanism of action is not fully known, Acetarsol may bind to protein-containing sulfhydryl groups located in the parasite, which then creates lethal As-S bonds, which then kills the parasite. [3]
Chemistry and Pharmacokinetics
Acetarsol has the molecular formula N-Acetyl-4-hydroxy-m-arsinillic acid, and it is a pentavalent arsenical compound with antiprotozoal and antihelmithic properties.The arsenic found in acetarsol is excreted mainly in urine. The level of arsenic after acetarsol administration reaches close to the toxic range in urine. [3] Some reports indicate a remission of arsenic which can be physiologically dangerous. [3]
Toxicity
Some reports indicate that Acetarsol can produce effects in the eyes such as optic neuritis and optic atrophy.[7]
References
- ↑ "FDA Substance Registration System: Acetarsol". Retrieved 6 May 2021.
- ↑ Chen MY, Smith NA, Fox EF, Bingham JS, Barlow D (April 1999). "Acetarsol pessaries in the treatment of metronidazole resistant Trichomonas vaginalis". Int J STD AIDS. 10 (4): 277–80. doi:10.1258/0956462991913943. PMID 12035784. S2CID 27353282.
- 1 2 3 4 5 6 PubChem. "Acetarsol". pubchem.ncbi.nlm.nih.gov. Retrieved 2021-03-31.
- ↑ Éric Fouassier, Ces poisons qui guérissent, oct. 1996, p. 5.
- ↑ Traité de chimie organique, sous la direction de Victor Grignard, Paul Baud, vol. 22, Masson, 1959, p. 1127-1130.
- ↑ Gionchetti P, Rizzello F, Morselli C, Campieri M (October 2004). "Review article: problematic proctitis and distal colitis". Aliment. Pharmacol. Ther. 20 Suppl 4: 93–6. doi:10.1111/j.1365-2036.2004.02049.x. PMID 15352902. S2CID 72699260.
- ↑ PubChem. "Acetarsol". pubchem.ncbi.nlm.nih.gov. Retrieved 2022-01-16.