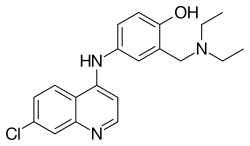
Amodiaquine
 | |
| Names | |
|---|---|
| Trade names | Amdaquine, Amobin, others[1] |
IUPAC name
| |
| Clinical data | |
| Main uses | Malaria[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Defined daily dose | 500 mg[3] |
| External links | |
| AHFS/Drugs.com | International Drug Names |
| Chemical and physical data | |
| Formula | C20H22ClN3O |
| Molar mass | 355.861 g/mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Amodiaquine (ADQ) is a medication used to treat malaria, including Plasmodium falciparum malaria when uncomplicated.[4][5] It is recommended to be given with artesunate to reduce the risk of resistance.[4] Due to the risk of rare but serious side effects, it is not generally recommended to prevent malaria.[4] Though, the WHO in 2013 recommended use for seasonal preventive in children at high risk in combination with sulfadoxine and pyrimethamine.[6]
The side effects of amodiaquine are generally minor to moderate and are similar to those of chloroquine.[5] Rarely liver problems or low blood cell levels may occur.[4] When taken in excess headaches, trouble seeing, seizures, and cardiac arrest may occur.[4] While not extensively studied as of 2007, it appeared to be safe in pregnancy.[7] Amodiaquine is a 4-aminoquinoline compound related to chloroquine.[4]
Amodiaquine was first made in 1948.[8] It is on the World Health Organization's List of Essential Medicines.[9] The wholesale cost is about US$0.01 per dose as of 2014.[10] While not available in the United States,[11] it is widely available in Africa.[4][12]
Medical uses
Amodiaquine has become an important drug in the combination therapy for malaria treatment in Africa.[13] It is often used in combination with artensunate as a by mouth artemisinin-based combination therapy (ACT) for uncomplicated P. falciparum malaria.[14] Amodiaquine has also been found to work against chloroquine-resistant P. falciparum strains of malaria.[15]
It is also used in combination with sulfadoxine/pyrimethamine.[16][17]
Dosage
The defined daily dose is 500 mg (by mouth).[3] It has been used at 10 mg of the base per kg once per day for three days.[2]
Interactions
There have been reports of increased liver toxicity in people with HIV/AIDS on zidovudine or efavirenz when treated with amodiaquine-containing ACT regimens, therefore it is recommended that these people avoid amodiaquine.[14]
Pharmacokinetics and pharmacogenetics
It is bioactivated hepatically to its primary metabolite, N-desethylamodiaquine, by the cytochrome p450 enzyme CYP2C8. Among amodiaquine users, several rare but serious side effects have been reported and linked to variants in the CYP2C8 alleles. CYP2C8*1 is characterized as the wild-type allele, which shows an acceptable safety profile, while CYP2C8*2, *3 and *4 all show a range of "poor metabolizer" phenotypes. People who are poor metabolizers of amodiaquine display lower treatment efficacy against malaria, as well as increased toxicity.[18] Several studies have been conducted to determine the prevalence of CYP2C8 alleles amongst malaria patients in East Africa, and have tentatively shown the variant alleles have significant prevalence in that population.[19] About 3.6% of the population studied showed high risk for a poor reaction to or reduced treatment outcomes when treated with amodiaquine. This information is useful in developing programs of pharmacovigilance in East Africa, and have important clinical considerations for prescribing antimalarial medications in regions with high CYP2C8 variant frequency.
See also
- Structurally similar is FGI-104
References
- ↑ "Amodiaquine". drugs.com. Archived from the original on 27 November 2016. Retrieved 27 November 2016.
- 1 2 "AMODIAQUINE = AQ oral - Essential drugs". medicalguidelines.msf.org. Archived from the original on 24 January 2021. Retrieved 25 August 2020.
- 1 2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 25 July 2020. Retrieved 21 September 2020.
- 1 2 3 4 5 6 7 Nair, A; Abrahamsson, B; Barends, DM; Groot, DW; Kopp, S; Polli, JE; Shah, VP; Dressman, JB (December 2012). "Biowaiver monographs for immediate release solid oral dosage forms: amodiaquine hydrochloride". Journal of Pharmaceutical Sciences. 101 (12): 4390–401. doi:10.1002/jps.23312. PMID 22949374.
- 1 2 Olliaro, P; Mussano, P (2003). "Amodiaquine for treating malaria". The Cochrane Database of Systematic Reviews (2): CD000016. doi:10.1002/14651858.CD000016. PMC 6532704. PMID 12804382.
- ↑ Seasonal malaria chemoprevention with sulfadoxine–pyrimethamine plus amodiaquine in children: a field guide (PDF). Geneva: The World Health Organization. August 2013. ISBN 978 92 4 150473 7. Archived (PDF) from the original on 2020-07-26. Retrieved 2020-01-14.
- ↑ Tagbor, HK; Chandramohan, D; Greenwood, B (November 2007). "The safety of amodiaquine use in pregnant women". Expert Opinion on Drug Safety. 6 (6): 631–5. doi:10.1517/14740338.6.6.631. PMID 17967151.
- ↑ Profiles of Drug Substances, Excipients and Related Methodology. Academic Press. 1992. p. 45. ISBN 9780080861166. Archived from the original on 2017-09-08.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ "Amodiaquine". International Drug Price Indicator Guide. Archived from the original on 29 March 2019. Retrieved 4 December 2015.
- ↑ "Amodiaquine". Livertox. Archived from the original on 27 November 2016. Retrieved 27 November 2016.
- ↑ Centers for Disease Control, (CDC). (12 April 1985). "Revised recommendations for preventing malaria in travelers to areas with chloroquine-resistant Plasmodium falciparum". MMWR. Morbidity and Mortality Weekly Report. 34 (14): 185–90, 195. PMID 3156271.
- ↑ Kerb, Reinhold; Fux; Morike; Kremsner; Gil; Gleiter; Schwab (2009). "Pharmacogenetics of antimalarial drugs: effect on metabolism and transport". Lancet Infectious Diseases. 9 (12): 760–774. doi:10.1016/S1473-3099(09)70320-2. PMID 19926036.
- 1 2 World Health Organization (2015). Guidelines for the treatment of malaria (3rd ed.). World Health Organization. hdl:10665/162441. ISBN 9789241549127.
- ↑ "amodiaquine". Pubchem. Archived from the original on 2016-10-29. Retrieved 2016-10-28.
- ↑ Staedke SG, Kamya MR, Dorsey G, Gasasira A, Ndeezi G, Charlebois ED, et al. (August 2001). "Amodiaquine, sulfadoxine/pyrimethamine, and combination therapy for treatment of uncomplicated falciparum malaria in Kampala, Uganda: a randomised trial". Lancet. 358 (9279): 368–74. doi:10.1016/S0140-6736(01)05557-X. PMID 11502317.
- ↑ World Health Organization (2013). Seasonal malaria chemoprevention with sulfadoxine–pyrimethamine plus amodiaquine in children: a field guide. World Health Organization. hdl:10665/85726. ISBN 9789241504737.
- ↑ Elyazar, Iqbal; Hay, Baird (April 2011). Malaria Distribution, Prevalence, Drug Resistance, and control in Indonesia. Advances in Parasitology. Vol. 74. pp. 41–175. doi:10.1016/B978-0-12-385897-9.00002-1. ISBN 9780123858979. PMC 3075886. PMID 21295677. Archived from the original on 2017-09-23. Retrieved 2018-07-26.
- ↑ Roederer, Mary; Mcleod, Juliano (2011). "Can pharmacogenetics improve malaria". Bulletin of the World Health Organization. 89 (11): 838–845. doi:10.2471/BLT.11.087320. PMC 3209725. PMID 22084530.
External links
| External sites: |
|
|---|---|
| Identifiers: |