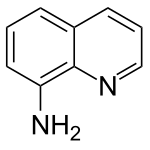
8-Aminoquinoline
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Quinolin-8-amine | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.008.572 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C9H8N2 |
| Molar mass | 144.177 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
8-Aminoquinoline is the 8-amino derivative of quinoline. It is structurally analogous to 8-hydroxyquinoline. The two nitrogen atoms are ideally situated to form complexes with metal ions. Derivatives of 8-aminoquinoline are effective directing groups in organic synthesis.[1][2]
Derivatives
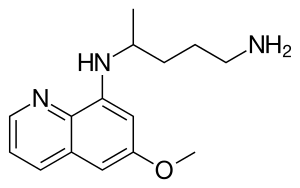
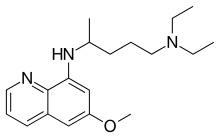
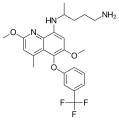
The derivatives primaquine, tafenoquine and pamaquine have been tested for anti-malaria activity.[3][4] Primaquine is still used routinely worldwide as part of the treatment of Plasmodium vivax and Plasmodium ovale malaria. Tafenoquine was approved for medical use in Australia and in the United States in 2018.[5][6]
References
- ↑ Daugulis, Olafs; Roane, James; Tran, Ly Dieu (2015). "Bidentate, Monoanionic Auxiliary-Directed Functionalization of Carbon–Hydrogen Bonds". Accounts of Chemical Research. 48 (4): 1053–1064. doi:10.1021/ar5004626. PMC 4406856. PMID 25756616.
- ↑ Corbet, Matthieu; De Campo, Floryan (2013). "8-Aminoquinoline: A Powerful Directing Group in Metal-Catalyzed Direct Functionalization of C-H Bonds". Angewandte Chemie International Edition. 52 (38): 9896–9898. doi:10.1002/anie.201303556. PMID 23939922.
- ↑ Nqoro, Xhamla; Tobeka, Naki; Aderibigbe, Blessing (2017). "Quinoline-Based Hybrid Compounds with Antimalarial Activity". Molecules. 22 (12): 2268. doi:10.3390/molecules22122268. PMC 6149725. PMID 29257067.
- ↑ Sweeney AW; Blackburn CRB; KH Rieckmann (1 August 2004). "Short report: The activity of pamaquine, an 8-aminoquinoline drug, against sporozoite-induced infections of Plasmodium vivax (New Guinea strains)". Am J Trop Med Hyg. 71 (2): 187–189. doi:10.4269/ajtmh.2004.71.2.0700187. PMID 15306708.
- ↑ Haston JC, Hwang J, Tan KR (November 2019). "Guidance for Using Tafenoquine for Prevention and Antirelapse Therapy for Malaria — United States, 2019" (PDF). MMWR. Morbidity and Mortality Weekly Report. 68 (46): 1062–1068. doi:10.15585/mmwr.mm6846a4. PMC 6871897. PMID 31751320.
- ↑ Hounkpatin, Aurore B; Kreidenweiss, Andrea; Held, Jana (March 2019). "Clinical utility of tafenoquine in the prevention of relapse of Plasmodium vivax malaria: a review on the mode of action and emerging trial data". Infection and Drug Resistance. 12: 553–570. doi:10.2147/IDR.S151031. PMC 6411314. PMID 30881061.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.