Primaquine
 | |
| Names | |
|---|---|
| Other names | primaquine phosphate |
IUPAC name
| |
| Clinical data | |
| Main uses | Treat and prevent malaria, Pneumocystis pneumonia[1] |
| Side effects | Nausea, vomiting, stomach cramps[1][2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Routes of use | By mouth |
| Defined daily dose | 15 milligrams[3] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607037 |
| Legal | |
| License data |
|
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | 96%[4] |
| Metabolism | Liver |
| Elimination half-life | 6 hours |
| Chemical and physical data | |
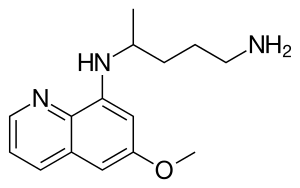
| Formula | C15H21N3O |
| Molar mass | 259.347 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
SMILES
| |
InChI
| |
Primaquine is a medication used to treat and prevent malaria and to treat Pneumocystis pneumonia.[1] Specifically it is used for malaria due to Plasmodium vivax and Plasmodium ovale along with other medications and for prevention if other options cannot be used.[1] It is an alternative treatment for Pneumocystis pneumonia together with clindamycin.[1][5] It is taken by mouth.[1]
Common side effects include nausea, vomiting, and stomach cramps.[1][2] Primaquine should not be given to people with glucose-6-phosphate dehydrogenase (G6PD) deficiency due to the risk of red blood cell breakdown.[2] It is often recommended that primaquine not be used during pregnancy.[6][7] It may be okay in breastfeeding when the baby is known not to have G6PD deficiency.[7] The mechanisms of action is not entirely clear but is believed to involve effects on the malaria parasites' DNA.[1]
Primaquine was first made in 1946.[5] It is on the World Health Organization's List of Essential Medicines.[8] It is available as a generic medication.[1] The wholesale cost in the developing world is US$0.04–0.11 per day.[9] In the United States a typical course of treatment is $50–100.[6]
Medical uses
Malaria
Primaquine is primarily used to prevent relapse of malaria due to Plasmodium vivax and Plasmodium ovale.[10] It eliminates hypnozoites, the dormant liver form of the parasite,[11] after the organisms have been cleared from the bloodstream.[10] If primaquine is not administered to patients with proven P. vivax or P. ovale infection, a very high likelihood of relapse exists for weeks or months (sometimes years).[10] Use in combination with quinine or chloroquine each of which is very effective at clearing P. vivax from blood, improves outcomes; they appear to also potentiate the action of primaquine.[12]
As of 2016, the US Centers for Disease Control and Prevention recommended the use of primaquine for primary prophylaxis prior to travel to areas with a high incidence of P. vivax, and for terminal prophylaxis (anti-relapse therapy) after travel.[2]
A single dose of primaquine has rapid and potent ability to kill gametocytes (stage V) of P. falciparum and P. vivax in blood; it also kills asexual trophozoites of P. vivax in blood, but not of P. falciparum.[12] Because of its action against gametocytes, the WHO recommends it for use in reducing transmission to control P. falciparum infections.[13]
Pneumocystis pneumonia
Primaquine is also used in the treatment of Pneumocystis pneumonia (PCP), a fungal infection commonly occurring in people with AIDS and, more rarely, in those taking immunosuppressive drugs. To treat PCP effectively, it is usually combined with clindamycin.[5]
Special populations
Primaquine has not been studied extensively in people 65 and older so it is not known if dosing should be adjusted for this population.[14]
Primaquine should not be administered to anyone with G6PD deficiency because a severe reaction can occur, resulting in hemolytic anemia.[2] However, the WHO has recommended that a single dose of primaquine (0.25 mg/kg) is safe to give even in individuals with G6PD deficiency, for the purpose of preventing transmission of P. falciparum malaria.[13]
Primaquine is contraindicated in pregnancy, because the glucose-6-phosphate dehydrogenase status of the fetus would be unknown.[2]
Primaquine overdose can cause a dangerous reduction in various blood cell counts, and therefore should be avoided in people at risk for agranulocytosis, which include people with conditions such as rheumatoid arthritis and lupus erythematosus, and those taking concurrent medications that also decrease blood cell counts.[14]
Dosage
The defined daily dose is 15 milligrams (by mouth).[3]
Side effects
Common side effects of primaquine administration include nausea, vomiting, and stomach cramps.[2][14]
In persons with cytochrome b5 reductase deficiency, primaquine causes methemoglobinemia, a condition in which the blood carries less oxygen that it does normally.[14]
Overdosing can reduce the number of function of various kinds of blood cells, including loss of red blood cells, methemoglobinemia, and loss of white blood cells.[14]
Persons with glucose-6-phosphate dehydrogenase deficiency (G6PD) may develop hemolytic anemia from primaquine.[15]
Pharmacology
Mechanism of action
Primaquine is lethal to P. vivax and P. ovale in the liver stage, and also to P. vivax in the blood stage through its ability to do oxidative damage to the cell. However, the exact mechanism of action is not fully understood.[7]
Pharmacokinetics
Primaquine is well-absorbed in the gut and extensively distributed in the body without accumulating in red blood cells. Administration of primaquine with food or grapefruit juice increases its oral bioavailibity.[16] In blood, about 20% of circulating primaquine is protein-bound, with preferential binding to the acute phase protein orosomucoid. With a half-life on the order of 6 hours, it is quickly metabolized by liver enzymes to carboxyprimaquine, which does not have anti-malarial activity. Renal excretion of the parent drug is less than 4%.[7][17]
Chemistry
Primaquine is an analog of pamaquine which was the first drug of the 8-aminoquinoline class; tafenoquine is another such drug.[12]
History
Primaquine was first made by Robert Elderfield of Columbia University in the 1940s as part of a coordinated effort led by the Office of Scientific Research and Development in World War II to develop anti-malarial drugs to protect and treat soldiers fighting in the Pacific theater.[12][18]
Society and culture
It is on the World Health Organization's List of Essential Medicines.[8]
It is a generic drug and is available under many brand names worldwide, including Jasoprim, Malirid, Neo-Quipenyl, Pimaquin, Pmq, Primachina, Primacin, Primaquina, Primaquine, Primaquine diphosphate, Primaquine Phosphate, and Remaquin.[19]
Synonyms
Research
Primaquine has been studied in animal models of Chagas disease and was about four times as effective as the standard of care, nifurtimox.[5]
References
- 1 2 3 4 5 6 7 8 9 "Primaquine Phosphate". The American Society of Health-System Pharmacists. Archived from the original on 20 December 2016. Retrieved 2 December 2016.
- 1 2 3 4 5 6 7 Arguin, Paul M.; Tan, Kathrine R. (2016). "Malaria - Chapter 3". In Brunette, Gary W. (ed.). CDC Health Information for International Travel 2016 (Yellow Book). CDC and Oxford University Press. ISBN 978-0-19-937915-6. Archived from the original on 2016-01-14.
- 1 2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 20 September 2020. Retrieved 21 September 2020.
- ↑ Mihaly GW, Ward SA, Edwards G, et al. (1985). "Pharmacokinetics of primaquine in man. I. Studies of the absolute bioavailability and effects of dose size". Br J Clin Pharmacol. 19 (6): 745–50. doi:10.1111/j.1365-2125.1985.tb02709.x. PMC 1463857. PMID 4027117.
- 1 2 3 4 Vale, Nuno; Moreira, Rui; Gomes, Paula (March 2009). "Primaquine revisited six decades after its discovery". European Journal of Medicinal Chemistry. 44 (3): 937–953. doi:10.1016/j.ejmech.2008.08.011. hdl:10216/82052. PMID 18930565.
- 1 2 Hamilton, Richart (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 57. ISBN 9781284057560.
- 1 2 3 4 Hill, D. R.; Baird, J. K.; Parise, M. E.; Lewis, L. S.; Ryan, E. T.; Magill, A. J. (2006). "Primaquine: Report from CDC expert meeting on malaria chemoprophylaxis I". The American Journal of Tropical Medicine and Hygiene. 75 (3): 402–15. doi:10.4269/ajtmh.2006.75.402. PMID 16968913. Archived from the original on 2014-01-23.
- 1 2 World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ "Primaquine". International Drug Price Indicator Guide. Archived from the original on 8 October 2018. Retrieved 3 December 2016.
- 1 2 3 "Treatment of Malaria (Guidelines For Clinicians)" (PDF). CDC. July 2013. Archived (PDF) from the original on 2017-08-29.
- ↑ Markus, MB (2011). "Malaria: Origin of the Term "Hypnozoite"". Journal of the History of Biology. 44 (4): 781–786. doi:10.1007/s10739-010-9239-3. PMID 20665090.
- 1 2 3 4 Baird JK, Rieckmann KH (March 2003). "Can primaquine therapy for vivax malaria be improved?". Trends Parasitol. 19 (3): 115–20. doi:10.1016/S1471-4922(03)00005-9. PMID 12643993. Archived from the original on 2018-07-23. Retrieved 2019-09-19.
- 1 2 Single dose primaquine as a gametocytocide in Plasmodium falciparum malaria. Geneva, Switzerland: World Health Organization. October 2012. Archived from the original on 2 January 2014. Retrieved 2 January 2014.
- 1 2 3 4 5 "Primaquine label" (PDF). FDA. November 2007. Archived (PDF) from the original on 10 November 2016. Retrieved 10 November 2016.
- ↑ Keystone, Jay S.; Kozarsky, Phyllis E.; Connor, Bradley A.; Nothdurft, Hans D.; Mendelson, Marc; Leder, Karin (2018). Travel Medicine E-Book. Elsevier Health Sciences. p. 185. ISBN 9780323547710. Archived from the original on 2020-08-19. Retrieved 2020-06-04.
- ↑ Cuong BT, Binh VQ, Dai B, et al. Does gender, food or grapefruit juice alter the pharmacokinetics of primaquine in healthy subjects? British Journal of Clinical Pharmacology. 2006;61(6):682-689. doi:10.1111/j.1365-2125.2006.02601.x.
- ↑ Pubchem. "PRIMAQUINE | C15H21N3O - PubChem". pubchem.ncbi.nlm.nih.gov. Archived from the original on 2016-11-10. Retrieved 2016-11-09.
- 1 2 Edgcomb JH, Arnold J, Young EH, et al. (1950). "Primaquine, SN 13272, a new curative agent in vivax malaria; a preliminary report". Journal of the National Malaria Society. 9 (4): 285–92. PMID 14804087.
- ↑ "Primaquine brand names". Drugs.com. Archived from the original on 11 November 2016. Retrieved 10 November 2016.
- ↑ Alving AS, Arnold J, Hockwald RS, Clayman CB, Dern RJ, Beutler E, Flanagan CL (1955). "Potentiation of the curative action of primaquine in vivax malaria by quinine and chloroquine". J Lab Clin Med. 46 (2): 301–6. PMID 13242948.
{{cite journal}}: CS1 maint: uses authors parameter (link)
External links
| External sites: |
|
|---|---|
| Identifiers: |