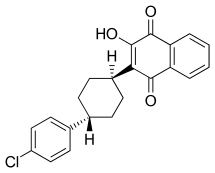
Atovaquone
 | |
 | |
| Names | |
|---|---|
| Trade names | Mepron |
IUPAC name
| |
| Clinical data | |
| Drug class | Antiprotozoal[1] |
| Main uses | Pneumocystis jirovecii pneumonia (PCP), toxoplasmosis, babesiosis[2] |
| Side effects | Headache, fever, anxiety, trouble sleeping, vivid dreams, nausea, diarrhea, skin rash, itching[3] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a693003 |
| Legal | |
| Legal status | |
| Pharmacokinetics | |
| Elimination half-life | 2.2–3.2 days |
| Chemical and physical data | |
| Formula | C22H19ClO3 |
| Molar mass | 366.84 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 216 to 219 °C (421 to 426 °F) |
SMILES
| |
InChI
| |
Atovaquone, sold under the brand name Mepron, is an medication used to treat and prevent Pneumocystis jirovecii pneumonia (PCP), toxoplasmosis and babesiosis.[2][1] For PCP it is used in those who cannot take trimethoprim/sulfamethoxazole.[2] It is taken by mouth.[2]
Common side effects include headache, fever, anxiety, trouble sleeping, vivid dreams, nausea, diarrhea, skin rash, and itching.[3] Other side effects may include liver problems and angioedema.[2] Safety in pregnancy and breastfeeding is unclear.[5] It is a quinone, specifically a naphthoquinone.[3][2]
Atovaquone was approved for medical use in the United States in 1992.[3] It is available as a generic medication.[6] In the United Kingdom 50 doses of 750 mg costs the NHS about £470 as of 2021.[6] This amount in the United States is about 220 USD.[7]
Medical uses
Atovaquone is a medication used to treat or prevent:
- For pneumocystis pneumonia (PCP),[8][9] it is used in mild cases, although it is not approved for treatment of severe cases.
- For toxoplasmosis,[10] the medication has antiparasitic and therapeutic effects.
- For babesia, it is often used in conjunction with oral azithromycin.[11]
Trimethoprim/sulfamethoxazole (TMP-SMX) is generally considered first-line therapy for PCP (not to be confused with sulfadiazine and pyrimethamine, which is first line for toxoplasmosis). However, atovaquone may be used in patients who cannot tolerate, or are allergic to, sulfonamide medications such as TMP-SMX. In addition, atovaquone has the advantage of not causing myelosuppression, which is an important issue in patients who have undergone bone marrow transplantation.
Atovaquone is given prophylactically to kidney transplant patients to prevent PCP in cases where TMP-SMX is contraindicated.
Dosage
For prevention it is used at a dose of 1,500 mg once per day while for treatment 750 mg twice per day is used for 21 days.[2]
Combinations
Atovaquone, as part of the combination atovaquone/proguanil with proguanil, is used to treatment and prevention of malaria. It has fewer side effects but is more expensive than mefloquine.[12] Resistance has been observed.[13]
Veterinary use
Atovaquone is used in livestock veterinary cases of babesiosis in cattle, especially if imidocarb resistance is a concern.[14]
Research
Preliminary research found that atovaquone could inhibit the replication of SARS-CoV-2 in vitro.[15]
Atovaquone has also been found to inhibit human coronavirus OC43 and feline coronavirus in vitro.[16]
References
- 1 2 "Atovaquone Monograph for Professionals". Drugs.com. Archived from the original on 3 June 2021. Retrieved 16 January 2022.
- 1 2 3 4 5 6 7 "Atovaquone Oral SUSPENSION- atovaquone suspension". DailyMed. 10 December 2019. Archived from the original on 7 August 2020. Retrieved 18 September 2020.
- 1 2 3 4 "Atovaquone". LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. National Institute of Diabetes and Digestive and Kidney Diseases. 2012. Archived from the original on 6 May 2021. Retrieved 16 January 2022.
- ↑ "Wellvone 750mg/5ml oral suspension - Summary of Product Characteristics (SmPC)". (emc). 28 November 2019. Archived from the original on 24 October 2020. Retrieved 18 September 2020.
- ↑ "Atovaquone (Mepron) Use During Pregnancy". Drugs.com. Archived from the original on 28 November 2020. Retrieved 16 January 2022.
- 1 2 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 641. ISBN 978-0857114105.
- ↑ "Atovaquone Prices and Atovaquone Coupons - GoodRx". GoodRx. Retrieved 16 January 2022.
- ↑ Hughes W, Leoung G, Kramer F, et al. (May 1993). "Comparison of atovaquone (566C80) with trimethoprim-sulfamethoxazole to treat Pneumocystis carinii pneumonia in patients with AIDS". N. Engl. J. Med. 328 (21): 1521–7. doi:10.1056/NEJM199305273282103. PMID 8479489.
- ↑ Dohn MN, Weinberg WG, Torres RA, et al. (August 1994). "Oral atovaquone compared with intravenous pentamidine for Pneumocystis carinii pneumonia in patients with AIDS. Atovaquone Study Group". Ann. Intern. Med. 121 (3): 174–80. doi:10.7326/0003-4819-121-3-199408010-00003. PMID 7880228.
- ↑ Djurković-Djaković O, Milenković V, Nikolić A, Bobić B, Grujić J (December 2002). "Efficacy of atovaquone combined with clindamycin against murine infection with a cystogenic (Me49) strain of Toxoplasma gondii". J. Antimicrob. Chemother. 50 (6): 981–7. doi:10.1093/jac/dkf251. PMID 12461021. Archived from the original on 2020-05-01. Retrieved 2021-10-01.
- ↑ Krause PJ, Lepore T, Sikand VK, et al. (November 2000). "Atovaquone and azithromycin for the treatment of babesiosis". N. Engl. J. Med. 343 (20): 1454–8. doi:10.1056/NEJM200011163432004. PMID 11078770.
- ↑ Malarone: New Malaria Medication With Fewer Side-effects Archived May 14, 2006, at the Wayback Machine
- ↑ Färnert A, Lindberg J, Gil P, et al. (March 2003). "Evidence of Plasmodium falciparum malaria resistant to atovaquone and proguanil hydrochloride: case reports". BMJ. 326 (7390): 628–9. doi:10.1136/bmj.326.7390.628. PMC 151974. PMID 12649236. Archived from the original on 2021-11-05. Retrieved 2021-10-01.
- ↑ Vial, Henri J.; Gorenflot, A. (2006). "Chemotherapy against babesiosis". Veterinary Parasitology. Elsevier. 138 (1–2): 147–160. doi:10.1016/j.vetpar.2006.01.048. ISSN 0304-4017.
- ↑ Farag A, Wang P, et al. (May 2020). "Identification of Atovaquone, Ouabain and Mebendazole as FDA-Approved Drugs Targeting SARS-CoV-2". chemRxiv (preprint). doi:10.26434/chemrxiv.12003930.v4. Archived from the original on 23 June 2020. Retrieved 20 June 2020.
- ↑ Wang CW, Peng TT, et al. (August 2020). "Repurposing old drugs as antiviral agents for coronaviruses". Biomedical Journal. 43 (4): 368–374. doi:10.1016/j.bj.2020.05.003. Archived from the original on 27 May 2020. Retrieved 22 October 2020.
External links
| External sites: |
|
|---|---|
| Identifiers: |