Metirosine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Demser |
| Other names | Metyrosine (USAN US) |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| License data |
|
| Pregnancy category |
|
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 3.4–3.7 hours |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.010.546 |
| Chemical and physical data | |
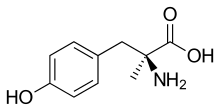
| Formula | C10H13NO3 |
| Molar mass | 195.218 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Metirosine (INN and BAN; α-Methyltyrosine, Metyrosine USAN, AMPT) is an antihypertensive drug. It inhibits the enzyme tyrosine hydroxylase and, therefore, catecholamine synthesis, which, as a consequence, depletes the levels of the catecholamines dopamine, adrenaline and noradrenaline in the body.
It is available as a generic medication.[1]
Clinical use
Metirosine has been shown to suppress catecholamine synthesis and alleviate symptoms related to catecholamine excess, including hypertension, headache, tachycardia, constipation, and tremor.[2] Metirosine is primarily used to reduce these symptoms in patients with pheochromocytoma.[3] It is contraindicated for the treatment of essential hypertension.
Metirosine is used as an off-label treatment for DiGeorge syndrome.[4]
Metirosine is used in scientific research to investigate the effects of catecholamine depletion on behavior.[5] There is evidence that catecholamine depletion causes an increase in sleepiness that is more pronounced than sleep deprivation, and that the fatigue lingers after the drug is discontinued. Negative mood is also a reported side effect of catecholamine depletion, although this is reported less consistently than sleepiness.[6]
See also
References
- ↑ "Metyrosine: FDA-Approved Drugs". U.S. Food and Drug Administration. Retrieved 15 August 2020.
- ↑ Naruse, Mitsuhide; Satoh, Fumitoshi; Tanabe, Akiyo; Okamoto, Takahiro; Ichihara, Atsuhiro; Tsuiki, Mika; Katabami, Takuyuki; Nomura, Masatoshi; Tanaka, Tomoaki (2018). "Efficacy and safety of metyrosine in pheochromocytoma/paraganglioma: a multi-center trial in Japan". Endocrine Journal. 65 (3): 359–371. doi:10.1507/endocrj.EJ17-0276. ISSN 0918-8959. PMID 29353821.
- ↑ Green KN, Larsson SK, Beevers DG, Bevan PG, Hayes B (August 1982). "Alpha-methyltyrosine in the management of phaeochromocytoma". Thorax. 37 (8): 632–3. doi:10.1136/thx.37.8.632. PMC 459390. PMID 7179194.
- ↑ "Doctors said the boy was suffering from teenage psychosis. What he really had was a rare genetic condition". The Washington Post. 30 April 2021.
- ↑ O'Leary, OF; Bechtholt, AJ; Crowley, JJ; Hill, TE; Page, ME; Lucki, I (2007). "Depletion of serotonin and catecholamines block the acute behavioral response to different classes of antidepressant drugs in the mouse tail suspension test". Psychopharmacology. 192 (3): 357–71. doi:10.1007/s00213-007-0728-9. PMID 17318507. S2CID 24850438.
- ↑ McCann, Una D.; Penetar, David M.; Shaham, Yavin; Thome, David R.; Sing, Helen C.; Thomas, Maria L.; Gillin, J. Christian; Belenky, Gregory (June 1993). "Effects of Catecholamine Depletion on Alertness and Mood in Rested and Sleep Deprived Normal Volunteers". Neuropsychopharmacology. 8 (4): 345–356. doi:10.1038/npp.1993.34. ISSN 0893-133X. PMID 8099791.
External links
- "Metyrosine". Drug Information Portal. U.S. National Library of Medicine.