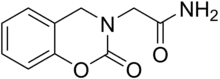
Caroxazone
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.038.481 |
| Chemical and physical data | |
| Formula | C10H10N2O3 |
| Molar mass | 206.201 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
Caroxazone (Surodil, Timostenil) is an antidepressant which was formerly used for the treatment of depression but is now no longer marketed.[1][2] It acts as a reversible monoamine oxidase inhibitor (RIMA) of both MAO-A and MAO-B subtypes, with five-fold preference for the latter.[3][4][5][6][7]
Synthesis
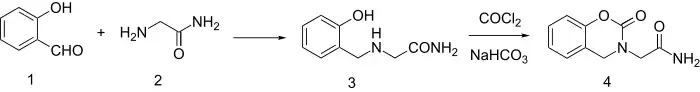
Synthesis starts by reductive amination of salicylaldehyde and glycinamide to give 3. The synthesis is completed by reaction with phosgene and NaHCO3.
See also
- Paraxazone, an isomer of Caroxazone
References
- ↑ Dictionary of organic compounds. London: Chapman & Hall. 1996. ISBN 0-412-54090-8.
- ↑ Cecchini S, Petri P, Ardito R, Bareggi SR, Torriti A (1978). "A comparative double-blind trial of the new antidepressant caroxazone and amitriptyline". The Journal of International Medical Research. 6 (5): 388–94. doi:10.1177/030006057800600507. PMID 359383. S2CID 40464443.
- ↑ Monoamine oxidase inhibitors in neurological diseases. New York: M. Dekker. 1994. ISBN 0-8247-9082-0.
- ↑ Moretti A, Caccia C, Martini A, Bonollo L, Amico A, Sega R, et al. (May 1981). "Effect of caroxazone, a new antidepressant drug, on monoamine oxidases in healthy volunteers". British Journal of Clinical Pharmacology. 11 (5): 511–5. doi:10.1111/j.1365-2125.1981.tb01158.x. PMC 1401585. PMID 7272163.
- ↑ Moretti A, Caccia C, Calderini G, Menozzi M, Amico A (October 1981). "Studies on the mechanism of action of caroxazone, a new antidepressant drug". Biochemical Pharmacology. 30 (19): 2728–31. doi:10.1016/0006-2952(81)90549-9. PMID 6170295.
- ↑ Martini A, Bonollo L, Nicolis FB, Sega R, Palermo A (June 1981). "Effects of caroxazone, a reversible monoamine oxidase inhibitor, on the pressor response to oral tyramine in man". British Journal of Clinical Pharmacology. 11 (6): 611–5. doi:10.1111/j.1365-2125.1981.tb01178.x. PMC 1402186. PMID 7272178.
- ↑ Martini A, Bonollo L, Nicolis FB, Sega R, Palermo A, Braibanti E (June 1981). "Effects of caroxazone, a reversible monoamine oxidase inhibitor, on the pressor response to intravenous tyramine in man". British Journal of Clinical Pharmacology. 11 (6): 605–10. doi:10.1111/j.1365-2125.1981.tb01177.x. PMC 1402193. PMID 7272177.
- ↑ Bernardi L, Coda S, Nicolella V, Vicario GP, Gioia B, Minghetti A, et al. (1979). "Radioisotopic and synthetic studies related to caroxazone metabolism in man". Arzneimittel-Forschung. 29 (9): 1412–6. PMID 583252.
- ↑ Bernardi L, Coda S, Pegrassi L, Suchowsky GK (August 1968). "Pharmacological properties of some derivatives of 1,3-benzoxazine". Experientia. 24 (8): 774–5. doi:10.1007/bf02144859. PMID 5683159. S2CID 30917127.
- ↑ Bernardi L, Coda S, Bonsignori A, Pegrassi L, Suchowsky GK (August 1969). "Central depressant properties of 3,1-benzoxazine derivates". Experientia. 25 (8): 787–8. doi:10.1007/bf01897874. PMID 5348526. S2CID 5347811.
- ↑ GB 1115759; L. Bernardi et al., U.S. Patent 3,427,313 (1965, 1969 both to Soc. Farma. Italia).
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.