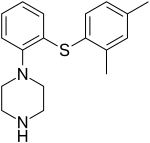
Vortioxetine
 | |
 | |
| Names | |
|---|---|
| Pronunciation | /vɔːrtiˈɒksətiːn/ vor-tee-OK-sə-teen |
| Trade names | Trintellix, Brintellix, others |
| Other names | Lu AA21004 |
IUPAC name
| |
| Clinical data | |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth (film-coated tablets) |
| Defined daily dose | 10 mg[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a614003 |
| Legal | |
| License data |
|
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | 75% (peak at 7–11 hours) |
| Protein binding | 98% |
| Metabolism | Extensive Liver, primarily CYP2D6-mediated oxidation |
| Elimination half-life | 66 hours |
| Excretion | 59% in urine, 26% in feces |
| Chemical and physical data | |
| Formula | C18H22N2S |
| Molar mass | 298.45 g/mol (379.36 as hydrobromide) g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Vortioxetine, sold under the trade names Trintellix and Brintellix among others, is a medication used to treat major depressive disorder.[3] Effectiveness is viewed as similar to that of other antidepressants.[3] In Britain, it is only recommended in people who have not improved sufficiently on two other antidepressants.[4] It is taken by mouth.[3]
Common side effects include constipation and nausea.[3] Serious side effects may include suicide in those under the age of 25, serotonin syndrome, bleeding, mania, and SIADH.[3] A withdrawal syndrome may occur if the dose is rapidly decreased.[3] Use during pregnancy and breastfeeding is not generally recommended.[4] It is classified as a serotonin modulator.[3] How it works is not entirely clear but is believed to be related to increasing serotonin levels.[3]
It was approved for medical use in the United States in 2013.[3] A month supply in the United Kingdom costs the NHS about £27.72 as of 2019.[4] In the United States the wholesale cost of this amount is about 368.40 USD.[5] In 2017, it was the 312th most commonly prescribed medication in the United States, with more than one million prescriptions.[6]
Medical uses
Vortioxetine is used as a treatment for major depressive disorder.[3] Effectiveness appear to be similar to other antidepressants.[3] It may be used when other treatments have failed.[7][8][9][10]
Vortioxetine is also used off label for anxiety.[11] A 2016 review found it was not useful in generalized anxiety disorder.[12]
Dosage
The defined daily dose is 10 mg by mouth.[2]
Side effects
The most common side effects reported with vortioxetine are nausea, diarrhea, dry mouth, constipation, vomiting, flatulence, dizziness, and sexual dysfunction.[7] However, with the singular exception of nausea, these side effects occurred in less than 10% of study participants given the active drug, with up to 8% of placebo-treated participants reporting the same side effects.
If vortioxetine is prescribed alongside traditional selective serotonin reuptake inhibitors (SSRIs), or other serotonergic drugs, this can induce the potentially life-threatening serotonin syndrome.[7]
Incidence of sexual dysfunction is only slightly higher in patients taking vortioxetine than in people taking placebos and occurs in less than 10% of vortioxetine-treated patients. As such, vortioxetine may be appropriate for people who have suffered sexual side effects from other antidepressant medications.[7][10]
Pharmacology
Pharmacodynamics
It increases serotonin concentrations in the brain by inhibiting its reuptake in the synapse, and by modulating (activating certain receptors while blocking, or antagonizing, others) certain serotonin receptors. This puts it in the class of atypical antidepressants known as serotonin modulators and stimulators.
Vortioxetine is a serotonin modulator and stimulator like vilazodone.[13] It has been shown to possess the following pharmacological actions:[7][14][15][16][17][18]
| Target | Affinity | Functional activity | Action | |
| Ki (nM) | IC50 / EC50 (nM) | IA (%) | ||
| SERT* | 1.6 | 5.4 | — | Inhibition |
| NET* | 113 | — | — | Inhibition |
| 5-HT1A* | 15 | 200 | 96 | Agonist |
| 5-HT1B* | 33 | 120 | 55 | Partial agonist |
| 5-HT1D* | 54 | 370 | — | Antagonist |
| 5-HT3* | 3.7 | 12 | — | Antagonist |
| 5-HT7* | 19 | 450 | — | Antagonist |
| β1 | 46[14] | — | — | — |
* Human isoforms
Pharmacokinetics
Vortioxetine reaches peak plasma concentration (Cmax) within 7 to 11 hours post-administration (Tmax), and its mean terminal half-life (T½) is ≈ 66 hours.
Steady-state mean Cmax values were 9, 18, and 33 ng/mL following doses of 5, 10, and 20 mg/day. Steady-state plasma concentrations are typically reached within two weeks.[7]
Vortioxetine's pKa values are determined to be 9.1 (± 0.1) and 3.0 (± 0.2) according to Australian Public Assessment Report for vortioxetine hydrobromide.[19]
History
Vortioxetine was discovered by scientists at Lundbeck who reported the rationale and synthesis for the drug (then called Lu AA21004) in a 2011 paper.[14][20]
In 2007, the compound was in Phase II clinical trials, and Lundbeck and Takeda entered into a partnership in which Takeda paid Lundbeck $40 million upfront, with promises of up to $345 million in milestone payments, and Takeda agreed to pay most of the remaining cost of developing the drug. The companies agreed to co-promote the drug in the US and Japan, and that Lundbeck would receive a royalty on all such sales. The deal included another drug candidate, tedatioxetine (Lu AA24530), and could be expanded to include two other Lundbeck compounds.[21]
Vortioxetine was approved by the U.S. FDA for the treatment of major depressive disorder (MDD) in adults in September 2013,[22] and it was approved in Europe later that year.[23]
Society and culture
It is made by the pharmaceutical companies Lundbeck and Takeda.[7]
Cost
A month supply in the United Kingdom costs the NHS about £27.72 as of 2019.[4] In the United States the wholesale cost of this amount is about 368.40 USD.[5] In 2017, it was the 312th most commonly prescribed medication in the United States, with more than one million prescriptions.[6]
.svg.png.webp) Vortioxetine costs (US)
Vortioxetine costs (US).svg.png.webp) Vortioxetine prescriptions (US)
Vortioxetine prescriptions (US)
Names
Vortioxetine was previously trademarked as Brintellix in the United States, but on May 2, 2016, the US FDA approved a name change to Trintellix in order to avoid confusion with the blood-thinning medication Brilinta (ticagrelor).[24]
References
- ↑ "TGA eBS - Product and Consumer Medicine Information Licence". www.ebs.tga.gov.au. Archived from the original on 29 April 2018. Retrieved 29 April 2018.
- 1 2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 21 January 2021. Retrieved 7 September 2020.
- 1 2 3 4 5 6 7 8 9 10 11 "Vortioxetine Hydrobromide Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 8 February 2018. Retrieved 18 March 2019.
- 1 2 3 4 British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 376. ISBN 9780857113382.
- 1 2 "NADAC as of 2019-02-27". Centers for Medicare and Medicaid Services. Archived from the original on 2019-03-06. Retrieved 3 March 2019.
- 1 2 "Vortioxetine Hydrobromide - Drug Usage Statistics". ClinCalc. Archived from the original on 21 June 2020. Retrieved 11 April 2020.
- 1 2 3 4 5 6 7 US Label Archived 2016-01-31 at the Wayback Machine Last updated July 2014 after review in September, 2014. Versions of label are available at FDA index page Archived 2020-07-30 at the Wayback Machine Page accessed January 19, 2016
- ↑ Connolly, KR; Thase, ME (2016). "Vortioxetine: a New Treatment for Major Depressive Disorder". Expert Opinion on Pharmacotherapy. 17 (3): 421–31. doi:10.1517/14656566.2016.1133588. PMID 26679430.
The authors suggest that vortioxetine is currently a good second-line antidepressant option and shows promise, pending additional long-term data, to become a first-line antidepressant option.
- ↑ Köhler, Stephan; Cierpinsky, Katharina; Kronenberg, Golo; Adli, Mazda (2016). "The serotonergic system in the neurobiology of depression: Relevance for novel antidepressants". Journal of Psychopharmacology. 30 (1): 13–22. doi:10.1177/0269881115609072. PMID 26464458.
- 1 2 Bobo, William; Kelliny, Marc; Croarkin, Paul; Moore, Katherine (2015). "Profile of vortioxetine in the treatment of major depressive disorder: An overview of the primary and secondary literature". Therapeutics and Clinical Risk Management. 11: 1193. doi:10.2147/TCRM.S55313. PMC 4542474. PMID 26316764.
- ↑ Pae, Chi-Un; Wang, Sheng-Min; Han, Changsu; Lee, Soo-Jung; Patkar, Ashwin A.; Masand, Praksh S.; Serretti, Alessandro (May 2015). "Vortioxetine, a multimodal antidepressant for generalized anxiety disorder: a systematic review and meta-analysis". Journal of Psychiatric Research. 64: 88–98. doi:10.1016/j.jpsychires.2015.02.017. ISSN 1879-1379. PMID 25851751.
- ↑ Fu, Jie; Peng, Lilei; Li, Xiaogang (2016-04-19). "The efficacy and safety of multiple doses of vortioxetine for generalized anxiety disorder: a meta-analysis". Neuropsychiatric Disease and Treatment. 12: 951–959. doi:10.2147/NDT.S104050. ISSN 1176-6328. PMC 4844447. PMID 27143896.
- ↑ "Lundbeck's "Serotonin Modulator and Stimulator" Lu AA21004: How Novel? How Good? - GLG News". Archived from the original on 2011-07-24.
- 1 2 3 Bang-Andersen B, Ruhland T, Jørgensen M, et al. (May 2011). "Discovery of 1-[2-(2,4-dimethylphenylsulfanyl)phenyl]piperazine (Lu AA21004): a novel multimodal compound for the treatment of major depressive disorder". Journal of Medicinal Chemistry. 54 (9): 3206–21. doi:10.1021/jm101459g. PMID 21486038.
- ↑ N. Moore; B. Bang-Andersen; L. Brennum; K. Fredriksen; S. Hogg; A. Mork; T. Stensbol; H. Zhong; C. Sanchez; D. Smith (August 2008). "Lu AA21004: a novel potential treatment for mood disorders". European Neuropsychopharmacology. 18 (Supplement 4): S321. doi:10.1016/S0924-977X(08)70440-1.
- ↑ Sanchez, C; Asin, KE; Artigas, F (1 January 2015). "Vortioxetine, a Novel Antidepressant with Multimodal Activity: Review of Preclinical and Clinical Data". Pharmacology & Therapeutics. 145: 43–57. doi:10.1016/j.pharmthera.2014.07.001. ISSN 1879-016X. PMID 25016186.
- ↑ Stahl, Stephen M. (2013-04-11). Stahl's Essential Psychopharmacology: Neuroscientific Basis and Practical Applications (4th ed.). Cambridge University Press. ISBN 978-1107686465.
- ↑ "BindingDB Search: BDBM50400902 1-(2-(2,4-dimethylphenylsulfanyl)phenyl)piperazine". BindingDB. Archived from the original on 7 December 2018. Retrieved 7 December 2018.
- ↑ "Australian Public Assessment Report for vortioxetine hydrobromide" (PDF). p. 11. Archived (PDF) from the original on 2017-08-01. Retrieved 2018-05-05.
- ↑ Sanchez, C; Asin, KE; Artigas, F (2015). "Vortioxetine, a novel antidepressant with multimodal activity: review of preclinical and clinical data". Pharmacol. Ther. 145: 43–57. doi:10.1016/j.pharmthera.2014.07.001. PMID 25016186.
- ↑ Daniel Beaulieu for First Word Pharma. September 5th, 2007 Lundbeck, Takeda enter strategic alliance for mood disorder, anxiety drugs Archived 2016-10-10 at the Wayback Machine
- ↑ FDA approves new drug to treat major depressive disorder Archived 2013-10-03 at the Wayback Machine, U.S. Food and Drug Administration Press Announcement.
- ↑ EMA Brintellix page at EMA site Archived 2016-01-26 at the Wayback Machine Page accessed January 19, 2016
- ↑ Commissioner, Office of the. "Safety Alerts for Human Medical Products - Brintellix (vortioxetine): Drug Safety Communication - Brand Name Change to Trintellix, to Avoid Confusion With Antiplatelet Drug Brilinta (ticagrelor)". www.fda.gov. Archived from the original on 2016-05-05. Retrieved 2016-05-02.
External links
| External sites: |
|
|---|---|
| Identifiers: |