Bromocriptine
 | |
 | |
| Names | |
|---|---|
| Trade names | Parlodel, Cycloset, others[1] |
| Other names | Bromocriptine mesilate, bromocriptine mesylate, 2-Bromoergocriptine; CB-154 |
IUPAC name
| |
| Clinical data | |
| Main uses | Prolactinoma, Parkinson's disease, acromegaly, neuroleptic malignant syndrome, type 2 diabetes, to stop milk production[2][3][4] |
| Side effects | Nausea, headache, tiredness, dizziness, constipation, sleepiness[3] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682079 |
| Legal | |
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | 28% of oral dose |
| Metabolism | Extensively liver-mediated |
| Elimination half-life | 12–14 hours |
| Excretion | 85% bile (feces), 2.5–5.5% urine |
| Chemical and physical data | |
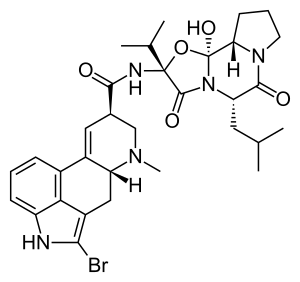
| Formula | C32H40BrN5O5 |
| Molar mass | 654.606 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Bromocriptine, sold under the brand name Parlodel among others, is a medication used to treat prolactinomas, Parkinson's disease, acromegaly, neuroleptic malignant syndrome, and stop milk production.[2][3] It may also be used in type 2 diabetes.[4] It is taken by mouth.[2]
Common side effects include nausea, headache, tiredness, dizziness, constipation, and sleepiness.[3] Other side effects may include low blood pressure, and pericardial effusions, confusion.[3] It has been used by many pregnant women without any evidence of harm to the baby.[5] It works by activating dopamine receptors and decreasing the release of prolactin.[2]
Bromocriptine was patented in 1968 and approved for medical use in 1975.[6] It is on the World Health Organization's List of Essential Medicines as an alternative to cabergoline.[7] It is available as a generic medication.[2] In the United Kingdom 30 pills of 2.5 mg cost the NHS about £75 as of 2021.[2] In the United States this amount costs about 30 USD.[8]
Medical uses
Bromocriptine is used to treat acromegaly and conditions associated with hyperprolactinemia like amenorrhea, infertility, hypogonadism, and prolactin-secreting adenomas. It is also used to prevent ovarian hyperstimulation syndrome[9][10][11] and to treat Parkinson's disease.[9]
Since the late 1980s it has been used, off-label, to reduce the symptoms of cocaine withdrawal but the evidence for this use is poor.[12]
A quick-release formulation of bromocriptine, is also used to treat type 2 diabetes.[13][14][15] When administered within 2 hours of awakening, it increases hypothalamic dopamine level and as a result decreases hepatic glucose production. It therefore acts as an adjunct to diet and exercise to improve glycemic control.[16]
Side effects
Most frequent side effects are nausea, orthostatic hypotension, headaches, and vomiting through stimulation of the brainstem vomiting centre.[17] Vasospasms with serious consequences such as myocardial infarction and stroke that have been reported in connection with the puerperium, appear to be extremely rare events.[18] Peripheral vasospasm (of the fingers or toes) can cause Raynaud's Phenomenon. Bromocriptine use has been anecdotally associated with causing or worsening psychotic symptoms (its mechanism is in opposition of most antipsychotics, whose mechanisms generally block dopamine receptors).[19] Pulmonary fibrosis has been reported when bromocriptine was used in high doses for the treatment of Parkinson's disease.[20]
Use to suppress milk production after childbirth was reviewed in 2014 and it was concluded that in this context a causal association with serious cardiovascular, neurological or psychiatric events could not be excluded with an overall incidence estimated to range between 0.005% and 0.04%. Additional safety precautions and stricter prescribing rules were suggested based on the data.[21][22] It is a bile salt export pump inhibitor.[23]
After long-term use of dopamine agonists, a withdrawal syndrome may occur during dose reduction or discontinuation with the following possible side effects: anxiety, panic attacks, dysphoria, depression, agitation, irritability, suicidal ideation, fatigue, orthostatic hypotension, nausea, vomiting, diaphoresis, generalized pain, and drug cravings. For some individuals, these withdrawal symptoms are short-lived and they make a full recovery, for others a protracted withdrawal syndrome may occur with withdrawal symptoms persisting for months or years.[24]
Pharmacology
| Site | Affinity (Ki [nM]) | Efficacy (Emax [%]) | Action |
|---|---|---|---|
| D1 | 692 | ? | ? |
| D2S | 5.0 | 41 | Partial agonist |
| D2L | 15 | 28 | Partial agonist |
| D3 | 6.8 | 68 | Partial agonist |
| D4 | 372 | 0 | Silent antagonist |
| D5 | 537 | ? | ? |
| 5-HT1A | 13 | 72 | Partial agonist |
| 5-HT1B | 355 | 66 | Partial agonist |
| 5-HT1D | 11 | 86 | Partial agonist |
| 5-HT2A | 107 | 69 | Partial agonist |
| 5-HT2B | 56 | 0 | Silent antagonist |
| 5-HT2C | 741 | 79 | Partial agonist |
| 5-HT6 | 33 | ? | ? |
| 5-HT7 | 11–126 | ? | ? |
| α1A | 4.2 | 0 | Silent antagonist |
| α1B | 1.4 | ? | ? |
| α1D | 1.1 | ? | ? |
| α2A | 11 | 0 | Silent antagonist |
| α2B | 35 | 0 | Silent antagonist |
| α2C | 28 | 0 | Silent antagonist |
| α2D | 68 | ? | ? |
| β1 | 589 | ? | ? |
| β2 | 741 | ? | ? |
| H1 | >10,000 | – | – |
| M1 | >10,000 | – | – |
| Notes: All receptors are human except α2D-adrenergic, which is rat (no human counterpart), and 5-HT7, which is rat/mouse.[25][28] | |||
Pharmacodynamics
Bromocriptine is a partial agonist of the dopamine D2 receptor.[25][26][29] It also interacts with other dopamine receptors and with various serotonin and adrenergic receptors.[25][26][27] Bromocriptine has additionally been found to inhibit the release of glutamate by reversing the GLT1 glutamate transporter.[30]
As an antagonist of the serotonin 5-HT2B receptor,[27] bromocriptine has not been associated with cardiac valvulopathy.[31] This is in contrast to other ergolines acting instead as 5-HT2B receptor agonists such as cabergoline and pergolide but is similar to lisuride which likewise acts as a 5-HT2B receptor antagonist.[31]
Chemistry
Like all ergopeptides, bromocriptine is a cyclol; two peptide groups of its tripeptide moiety are crosslinked, forming the >N-C(OH)< juncture between the two rings with the amide functionality.
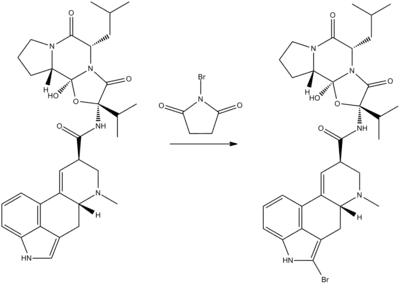
Bromocriptine is a semisynthetic derivative of a natural ergot alkaloid, ergocryptine (a derivative of lysergic acid), which is synthesized by bromination of ergocryptine using N-bromosuccinimide.[32][33]
History
Bromocriptine was discovered by scientists at Sandoz in 1965 and was first published in 1968; it was first marketed under the brand name Parlodel.[34][35]
A quick-release formulation of bromocriptine was approved by the FDA in 2009.[36]
Society and culture
Brand names
As of July 2017, bromocriptine was marketed under many brand names worldwide, including Abergin, Barlolin, Brameston, Brocriptin, Brom, Broma-Del, Bromergocryptine, Bromergon, Bromicon, Bromocorn, Bromocriptin, Bromocriptina, Bromocriptine, Bromocriptine mesilate, Bromocriptine mesylate, Bromocriptine methanesulfonate, Bromocriptini mesilas, Bromocriptinmesilat, Bromodel, Bromokriptin, Bromolac, Bromotine, Bromtine, Brotin, Butin, Corpadel, Cripsa, Criptine, Criten, Cycloset, Degala, Demil, Deparo, Deprolac, Diacriptin, Dopagon, Erenant, Grifocriptina, Gynodel, kirim, Kriptonal, Lactodel, Medocriptine, Melen, Padoparine, Palolactin, Parlodel, Pravidel, Proctinal, Ronalin, Semi-Brom, Serocriptin, Serocryptin, Suplac, Syntocriptine, Umprel, Unew, Updopa, Upnol B, and Volbro.[1]
As of July 2017 it was also marketed as a combination drug with metformin as Diacriptin-M, and as a veterinary drug under the brand Pseudogravin.[1]
References
- 1 2 3 "Bromocriptine international brand names". Drugs.com. Archived from the original on 6 August 2017. Retrieved 13 July 2017.
- 1 2 3 4 5 6 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 441. ISBN 978-0857114105.
- 1 2 3 4 5 "Bromocriptine Monograph for Professionals". Drugs.com. Archived from the original on 24 January 2021. Retrieved 12 January 2022.
- 1 2 "DailyMed - CYCLOSET- bromocriptine mesylate tablet". dailymed.nlm.nih.gov. Archived from the original on 29 March 2021. Retrieved 12 January 2022.
- ↑ "Bromocriptine Use During Pregnancy". Drugs.com. Archived from the original on 28 November 2020. Retrieved 12 January 2022.
- ↑ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 533. ISBN 9783527607495. Archived from the original on 2021-08-28. Retrieved 2021-06-30.
- ↑ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ↑ "Bromocriptine Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 7 November 2016. Retrieved 12 January 2022.
- 1 2 "Bromocriptine mesylate tablets -- original uses" (PDF). FDA. January 2012. Archived (PDF) from the original on 2017-02-28. For label updates see FDA index page for NDA 017962 Archived 2017-06-29 at the Wayback Machine
- ↑ Molitch ME (February 2017). "Diagnosis and Treatment of Pituitary Adenomas: A Review". JAMA. 317 (5): 516–524. doi:10.1001/jama.2016.19699. PMID 28170483. S2CID 205077946.
- ↑ Tang H, Mourad SM, Wang A, Zhai SD, Hart RJ (14 Apr 2021). "Dopamine agonists for preventing ovarian hyperstimulation syndrome". The Cochrane Database of Systematic Reviews. doi:10.1002/14651858.CD008605.pub4. PMID 33851429.
- ↑ Minozzi S, Amato L, Pani PP, Solimini R, Vecchi S, De Crescenzo F, et al. (May 2015). "Dopamine agonists for the treatment of cocaine dependence". The Cochrane Database of Systematic Reviews (5): CD003352. doi:10.1002/14651858.CD003352.pub4. PMC 6999795. PMID 26014366.
- ↑ "Bromocriptine mesylate tablet label" (PDF). FDA. February 2017. Archived (PDF) from the original on 2018-05-13.. For label updates see FDA index page for NDA 020866 Archived 2017-06-28 at the Wayback Machine
- ↑ Garber AJ, Blonde L, Bloomgarden ZT, Handelsman Y, Dagogo-Jack S (2013). "The role of bromocriptine-QR in the management of type 2 diabetes expert panel recommendations". Endocrine Practice. 19 (1): 100–6. doi:10.4158/EP12325.OR. PMID 23337160.
- ↑ Liang W, Gao L, Li N, Wang B, Wang L, Wang Y, et al. (October 2015). "Efficacy and Safety of Bromocriptine-QR in Type 2 Diabetes: A Systematic Review and Meta-Analysis". Hormone and Metabolic Research. 47 (11): 805–12. doi:10.1055/s-0035-1559684. PMID 26332757.
- ↑ Defronzo RA (April 2011). "Bromocriptine: a sympatholytic, d2-dopamine agonist for the treatment of type 2 diabetes". Diabetes Care. 34 (4): 789–94. doi:10.2337/dc11-0064. PMC 3064029. PMID 21447659. Archived from the original on 2021-11-04. Retrieved 2021-06-30.
- ↑ Weil C (1986). "The safety of bromocriptine in long-term use: a review of the literature". Current Medical Research and Opinion. 10 (1): 25–51. doi:10.1185/03007998609111089. PMID 3516579.
- ↑ Iffy L, McArdle JJ, Ganesh V, Hopp L (1996). "Bromocriptine related atypical vascular accidents postpartum identified through medicolegal reviews". Medicine and Law. 15 (1): 127–34. PMID 8691994.
- ↑ Boyd A (1995). "Bromocriptine and psychosis: a literature review". The Psychiatric Quarterly. 66 (1): 87–95. doi:10.1007/BF02238717. PMID 7701022. S2CID 29539691.
- ↑ Todman DH, Oliver WA, Edwards RL (1990). "Pleuropulmonary fibrosis due to bromocriptine treatment for Parkinson's disease". Clinical and Experimental Neurology. 27: 79–82. PMID 2129961.
- ↑ "European Medicines Agency - News and Events - CMDh endorses restricted use of bromocriptine for stopping breast milk production". www.ema.europa.eu. 2018-09-17. Archived from the original on 2014-08-28.
- ↑ "EMA rät vom Abstillmittel Bromocriptin ab". 2014-08-25. Archived from the original on 2015-06-09. Retrieved 2014-08-26. "EMA rät vom Abstillmittel Bromocriptin ab", article in Ärzteblatt
- ↑ Montanari F, Pinto M, Khunweeraphong N, Wlcek K, Sohail MI, Noeske T, et al. (January 2016). "Flagging Drugs That Inhibit the Bile Salt Export Pump" (PDF). Molecular Pharmaceutics. 13 (1): 163–71. doi:10.1021/acs.molpharmaceut.5b00594. PMID 26642869. Archived (PDF) from the original on 2021-11-04. Retrieved 2021-06-30.
- ↑ Nirenberg MJ (August 2013). "Dopamine agonist withdrawal syndrome: implications for patient care". Drugs & Aging. 30 (8): 587–92. doi:10.1007/s40266-013-0090-z. PMID 23686524. S2CID 207489653.
- 1 2 3 4 Millan MJ, Maiofiss L, Cussac D, Audinot V, Boutin JA, Newman-Tancredi A (November 2002). "Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. I. A multivariate analysis of the binding profiles of 14 drugs at 21 native and cloned human receptor subtypes". J Pharmacol Exp Ther. 303 (2): 791–804. doi:10.1124/jpet.102.039867. PMID 12388666.
- 1 2 3 Newman-Tancredi A, Cussac D, Audinot V, Nicolas JP, De Ceuninck F, Boutin JA, Millan MJ (November 2002). "Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. II. Agonist and antagonist properties at subtypes of dopamine D(2)-like receptor and alpha(1)/alpha(2)-adrenoceptor". J Pharmacol Exp Ther. 303 (2): 805–14. doi:10.1124/jpet.102.039875. PMID 12388667.
- 1 2 3 Newman-Tancredi A, Cussac D, Quentric Y, Touzard M, Verrièle L, Carpentier N, Millan MJ (November 2002). "Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. III. Agonist and antagonist properties at serotonin, 5-HT(1) and 5-HT(2), receptor subtypes". J Pharmacol Exp Ther. 303 (2): 815–22. doi:10.1124/jpet.102.039883. PMID 12388668.
- 1 2 National Institute of Mental Health. PDSP Ki Database (Internet). ChapelHill (NC): University of North Carolina. Available from: "Archived copy". Archived from the original on 2021-04-12. Retrieved 2021-04-12.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ de Leeuw van Weenen JE, Parlevliet ET, Maechler P, Havekes LM, Romijn JA, Ouwens DM, et al. (June 2010). "The dopamine receptor D2 agonist bromocriptine inhibits glucose-stimulated insulin secretion by direct activation of the alpha2-adrenergic receptors in beta cells". Biochemical Pharmacology. 79 (12): 1827–36. doi:10.1016/j.bcp.2010.01.029. PMID 20138024.
- ↑ Shirasaki Y, Sugimura M, Sato T (September 2010). "Bromocriptine, an ergot alkaloid, inhibits excitatory amino acid release mediated by glutamate transporter reversal". European Journal of Pharmacology. 643 (1): 48–57. doi:10.1016/j.ejphar.2010.06.007. PMID 20599932.
- 1 2 Cavero I, Guillon JM (2014). "Safety Pharmacology assessment of drugs with biased 5-HT(2B) receptor agonism mediating cardiac valvulopathy". J Pharmacol Toxicol Methods. 69 (2): 150–61. doi:10.1016/j.vascn.2013.12.004. PMID 24361689.
- ↑ E. Fluckiger, A. Hofmann, U.S. Patent 3,752,814 (1973)
- ↑ A. Hofmann, E. Flueckiger, DE 1926045 (1969)
- ↑ Sneader W, Corey EJ (2005). Drug Discovery: A History. John Wiley & Sons. p. 352. ISBN 9780471899792.
- ↑ Beekman AM, Barrow RA (2014). "Fungal Metabolites as Pharmaceuticals". Australian Journal of Chemistry. 67 (6): 827. doi:10.1071/CH13639.
- ↑ Holt RI, Barnett AH, Bailey CJ (December 2010). "Bromocriptine: old drug, new formulation and new indication". Diabetes, Obesity & Metabolism. 12 (12): 1048–57. doi:10.1111/j.1463-1326.2010.01304.x. PMID 20977575. S2CID 22908831.
External links
| Identifiers: |
|---|