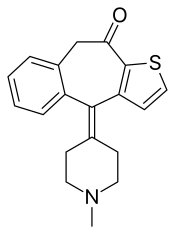
Ketotifen
 | |
| Names | |
|---|---|
| Trade names | Zaditor,[1] Zaditen[2] |
| Other names | Ketotifen fumarate |
IUPAC name
| |
| Clinical data | |
| Drug class | H1-antihistamine, mast cell stabilizer[3] |
| Main uses | Allergic conjunctivitis, allergic rhinitis[2][1] |
| Side effects | By mouth: Anxiety, trouble sleeping, irritability[2] Eye drops: Red eyes, runny nose, headache[3] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth (tablets), topical eye drops |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a604033 |
| Legal | |
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | 60% |
| Protein binding | 75% |
| Metabolism | Hepatic |
| Elimination half-life | 12 hours |
| Chemical and physical data | |
| Formula | C19H19NOS |
| Molar mass | 309.43 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Ketotifen, sold under the brand name Zaditor among others, is a medication used to treat allergic conjunctivitis and allergic rhinitis.[2][1] It is taken by mouth or applied as an eye drop.[2][1]
Common side effects, when taken by mouth, include anxiety, trouble sleeping, and irritability.[2] Common side effects, when used as eye drops, include redness of the eyes, runny nose, and headache.[3] While use is often not recommended, there is no evidence of harm in early pregnancy.[2] It is a H1-antihistamine and mast cell stabilizer.[3]
Ketotifen was patented in 1970 and came into medical use in 1976.[4] In the United Kingdom 60 pills of 1 mg costs the NHS about £8.[2] In the United States a 5 ml bottle of eye drops costs about 7 USD.[5]
Medical uses
Ketotifen relieves and prevents eye itchiness and/or irritation associated with most seasonal allergies. It starts working within minutes after administering the drops. The drug has not been studied in children under three.[1] The mean elimination half life is 12 hours.[6] Besides its anti-histaminic activity, it is also a functional leukotriene antagonist[7] and a phosphodiesterase inhibitor.[8]
"[O]ral ketotifen has been used in patients with asthma, allergic rhinitis, allergic conjunctivitis, atopic dermatitis, chronic urticaria, cold-induced urticaria, cholinergic urticaria, exercise-induced urticaria, [systemic mast cell disease including mastocytosis, MCAS, allergic and nonallergic anaphylaxis, angioedema], and food allergy in Canada, Europe, and Mexico." Now available via prescription at US compounding pharmacies: "For adults and older children with asthma or allergic disease, the recommended dose of ketotifen is 1 mg twice daily." "FDA staff did recommend more extensive evaluations for management of urticaria."[9]
Dosage
It may be used at a dose of 0.5 mg once per day to 1 mg twice per day.[2]
Side effects
Side effects include drowsiness, weight gain (11-12lbs), dry mouth, irritability, and increased nosebleeds.[10]
Pharmacology
Ketotifen is a selective antihistamine – that is, an inverse agonist of the histamine H1 receptor (Ki = 0.166 nM)[11] – and mast cell stabilizer.[12] In addition, ketotifen has weak anticholinergic (Ki = 204 nM for mACh) and antiserotonergic (Ki = 38.9 nM for 5-HT2A) activity.[11][13] However, at the dosages in which it is typically used clinically, both the anticholinergic and antiserotonergic activity of ketotifen are said not to be appreciable.[14]
Society and culture
Brand names
Ketotifen is marketed under many brand names worldwide.[15]
References
- 1 2 3 4 5 "Zaditor- ketotifen fumarate solution". DailyMed. 13 February 2020. Archived from the original on 11 June 2021. Retrieved 4 September 2020.
- 1 2 3 4 5 6 7 8 9 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 302. ISBN 978-0857114105.
- 1 2 3 4 "Ketotifen Monograph for Professionals". Drugs.com. Archived from the original on 11 June 2021. Retrieved 1 December 2021.
- ↑ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 548. ISBN 9783527607495. Archived from the original on 2020-08-13. Retrieved 2021-07-12.
- ↑ "Compare Ketotifen Prices - GoodRx". GoodRx. Archived from the original on 2020-11-23. Retrieved 2021-12-01.
- ↑ Grahnén, A.; Lönnebo, A.; Beck, O.; Eckernäs, S-Å; Dahlström, B.; Lindström, B. (1992). "Pharmacokinetics of ketotiffn after oral administration to healthy male subjects". Biopharmaceutics & Drug Disposition. 13 (4): 255–62. doi:10.1002/bdd.2510130404. PMID 1600111. S2CID 72293850.
- ↑ Fink, A.; Bibi, H.; Eliraz, A.; Schlesinger, M.; Bentwich, Z. (1986). "Ketotifen, disodium cromoglycate, and verapamil inhibit leukotriene activity: determination by tube leukocyte adherence inhibition assay". Annals of Allergy. 57 (2): 103–106. ISSN 0003-4738. PMID 3090908. Archived from the original on 2021-06-11. Retrieved 2021-07-12.
- ↑ Castillo, J. G.; Gamboa, P. M.; García, B. E.; Oehling, A. (1990). "Effect of ketotifen on phosphodiesterase activity from asthmatic individuals". Allergologia Et Immunopathologia. 18 (4): 197–201. ISSN 0301-0546. PMID 1702263. Archived from the original on 2021-06-11. Retrieved 2021-07-12.
- ↑ Sokol, Kristin C.; Amar, Neil K.; Starkey, Jonathan; Grant, J. Andrew (2013). "Ketotifen in the management of chronic urticaria: Resurrection of an old drug". Annals of Allergy, Asthma & Immunology. 111 (6): 433–6. doi:10.1016/j.anai.2013.10.003. PMC 4309375. PMID 24267353.
- ↑ "Zaditen - MIMS online". www.mims.co.uk. Archived from the original on 2020-10-25. Retrieved 2021-07-12.
- 1 2 Kakiuchi M, Ohashi T, Musoh K, Kawamura K, Morikawa K, Kato H (1997). "Studies on the novel antiallergic agent HSR-609: its penetration into the central nervous system in mice and guinea pigs and its selectivity for the histamine H1-receptor". Jpn. J. Pharmacol. 73 (4): 291–8. doi:10.1254/jjp.73.291. PMID 9165365.
- ↑ Thomas L. Lemke; David A. Williams (2008). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 1019–. ISBN 978-0-7817-6879-5. Archived from the original on 2021-06-11. Retrieved 2021-07-12.
- ↑ V Alagarsamy (16 June 2012). Textbook of Medicinal Chemistry Vol II - E-Book. Elsevier Health Sciences. pp. 38–. ISBN 978-81-312-3259-0. Archived from the original on 20 June 2021. Retrieved 12 July 2021.
- ↑ Jürgen Drews (6 December 2012). Immunopharmacology: Principles and Perspectives. Springer Science & Business Media. pp. 282–. ISBN 978-3-642-75561-3. Archived from the original on 18 June 2021. Retrieved 12 July 2021.
- ↑ "Ketotifen International". Drugs.com. Archived from the original on 11 April 2021. Retrieved 4 September 2020.
External links
| External sites: |
|
|---|---|
| Identifiers: |