Betahistine
 | |
 | |
| Names | |
|---|---|
| Trade names | Serc, others |
IUPAC name
| |
| Clinical data | |
| Main uses | Vertigo, ringing in the ears[1] |
| Side effects | Headache, nausea, abdominal discomfort[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth |
| Typical dose | 8 to 16 mg TID[1] |
| External links | |
| AHFS/Drugs.com | International Drug Names |
| Legal | |
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | ~100%[2] |
| Protein binding | <5%[2] |
| Metabolism | Liver[2] |
| Metabolites | • 2-(2-Aminoethyl)pyridine • 2-Pyridylacetic acid[2] |
| Elimination half-life | 3.5 hours[3] |
| Excretion | Urine: 91%[2] |
| Chemical and physical data | |
| Formula | C8H12N2 |
| Molar mass | 136.198 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Betahistine, sold under the brand name Serc among others, is a medication used for vertigo and ringing in the ears including that due to Ménière's disease.[1] Evidence for these uses; however, is weak.[4][5][6] Improvement may take a few days to occur.[3] It is taken by mouth.[1]
Common side effects include headache, nausea, and abdominal discomfort.[1] Safety in pregnancy and breastfeeding is unclear.[1] Betahistine is a H1 histamine agonist and a H3 histamine antagonist.[7] It is believed to work by improving blood flow to the inner ear.[3]
Betahistine was approved for medical use in the 1970s.[7] While approved in the United States in the 1970s, this approval was removed due to a lack of benefit.[7] It remains in use in other countries.[7] It is available as a generic medication.[1] In the United Kingdom 84 doses of 16 mg costs the NHS less than 5 pounds while in Canada it is about 25 CAD as of 2020.[1][8]
Medical uses
Betahistine is used to treat Ménière's disease and vertigo.[3] The supporting evidence for these uses is weak.[4][5][6] This includes there being no evidence regarding if betahistine prevents hearing loss or effects vertigo.[9]
While it is effective in BPPV, it is less effective than repositioning maneuvers.[8]
Dosage
The typical starting dose is 16 mg three times per day.[1] Long term a dose of 8 to 16 mg three times per day may be used.[1]
Side effects
People taking betahistine may experience following side effects:
- Headache
- Low level of gastric side effects
- Nausea can be a side effect, but the person is generally already experiencing nausea due to the vertigo so it goes largely unnoticed.
- People taking betahistine may experience several hypersensitivity and allergic reactions. In the November 2006 issue of "Drug Safety," Dr. Sabine Jeck-Thole and Dr. Wolfgang Wagner reported that betahistine may cause several allergic and skin-related side effects. These include rash in several areas of the body; itching and hives; and swelling of the face, tongue and mouth. Other hypersensitivity reactions reported include tingling, numbness, burning sensations, shortness of breath and laboured breathing. The study authors suggest that hypersensitivity reactions may be a direct result of betahistine's role in increasing histamine levels throughout the body. Hypersensitivity reactions quickly subside after betahistine has been discontinued.
Contraindications
Betahistine is contraindicated for people with pheochromocytoma. People with asthma or a history of peptic ulcer need to be closely monitored.
Digestive
Betahistine may also cause several digestive-related side effects. The package insert for Serc, a trade name for betahistine, states that people may experience several gastrointestinal side effects. These may include nausea, upset stomach, vomiting, diarrhea, dry mouth and stomach cramping. These symptoms are usually not serious and subside between doses. People experiencing chronic digestive problems may lower their dose to the minimum effective range and by taking betahistine with meals. Additional digestive problems may require that people consult their physician in order to find a possible suitable alternative.
Others
People taking betahistine may experience several other side effects ranging from mild to serious. The package insert states that people may experience nervous-system side effects, including headache. Some nervous system events may also partly be attributable to the underlying condition rather than the medication used to treat it. The study by Jeck-Thole and Wagner also reports that people may experience headache and liver problems, including increased liver enzymes and bile-flow disturbances. Any side effects that persist or outweigh the relief of symptoms of the original condition may warrant that the person consult their physician to adjust or change the medication.
Pharmacology
Pharmacodynamics
Betahistine is a strong antagonist of the histamine H3 receptor and a weak agonist of the histamine H1 receptor.[2]
Betahistine has two mechanisms of action. Primarily, it is a weak agonist on the H1 receptors located on blood vessels in the inner ear. This gives rise to local vasodilation and increased permeability, which helps to reverse the underlying problem of endolymphatic hydrops.
More importantly, betahistine has a powerful antagonistic effects at H3 receptors, thereby increasing the levels of neurotransmitters histamine, acetylcholine, norepinephrine, serotonin, and GABA released from the nerve endings. The increased amounts of histamine released from histaminergic nerve endings can stimulate receptors. This stimulation explains the potent vasodilatory effects of betahistine in the inner ear, that are well documented.
Betahistine seems to dilate the blood vessels within the inner ear which can relieve pressure from excess fluid and act on the smooth muscle.
It is postulated that betahistine's increase in the level of serotonin in the brainstem inhibits the activity of vestibular nuclei.
Pharmacokinetics
Betahistine comes in both a tablet form as well as an oral solution, and is taken orally. It is rapidly and completely absorbed. The mean plasma elimination half-life is 3 to 4 hours, and excretion is virtually complete in the urine within 24 hours. Plasma protein binding is very low. Betahistine is transformed into aminoethylpyridine and hydroxyethylpyridine and excreted with the urine as pyridylacetic acid. There is some evidence that one of these metabolites, aminoethylpyridine, may be active and exert effects similar to those of betahistine on ampullar receptors.[10]
Chemistry
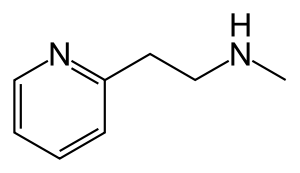
Betahistine chemically is 2-[2-(methylamino)ethyl]pyridine, and is formulated as the dihydrochloride salt. Its chemical structure closely resembles those of phenethylamine and histamine.
Society and culture
Brand names
Betahistine is marketed under a number of brand names, including Veserc, Serc, Hiserk, Betaserc, and Vergo.
Availability
Betahistine is widely used and available in Europe, including in the United Kingdom.[2] It was approved by the Food and Drug Administration in the early 1970s for Ménière's disease, but approval was later withdrawn for lack of evidence of efficacy. The withdrawal was upheld by a US court of appeals in 1968
References
- 1 2 3 4 5 6 7 8 9 10 11 BNF 79 : March 2020. London: Royal Pharmaceutical Society. 2020. p. 453. ISBN 9780857113658.
- 1 2 3 4 5 6 7 Anthony Dickenson (12 January 2017). Drugs in Neurology. Oxford University Press. pp. 408–409. ISBN 978-0-19-966436-8. Archived from the original on 28 August 2021. Retrieved 6 January 2018.
- 1 2 3 4 Adriana P. Tiziani (1 June 2013). Havard's Nursing Guide to Drugs. Elsevier Health Sciences. pp. 1063–. ISBN 978-0-7295-8162-2. Archived from the original on 28 August 2021. Retrieved 6 January 2018.
- 1 2 Murdin, L; Hussain, K; Schilder, AG (21 June 2016). "Betahistine for symptoms of vertigo". The Cochrane database of systematic reviews (6): CD010696. doi:10.1002/14651858.CD010696.pub2. PMID 27327415.
- 1 2 Wegner, I; Hall, DA; Smit, AL; McFerran, D; Stegeman, I (28 December 2018). "Betahistine for tinnitus". The Cochrane database of systematic reviews. 12: CD013093. doi:10.1002/14651858.CD013093.pub2. PMID 30908589.
- 1 2 James, AL; Burton, MJ (2001). "Betahistine for Menière's disease or syndrome". The Cochrane database of systematic reviews (1): CD001873. doi:10.1002/14651858.CD001873. PMID 11279734.
- 1 2 3 4 Babu, Seilesh; Schutt, Christopher A.; Bojrab, Dennis I. (2019). Diagnosis and Treatment of Vestibular Disorders. Springer. p. 192. ISBN 978-3-319-97858-1. Archived from the original on 2021-08-28. Retrieved 2020-10-07.
- 1 2 Ton, Joey (5 October 2020). "#274 Making your head spin: Betahistine for benign paroxysmal positional vertigo". CFPCLearn. Archived from the original on 28 March 2023. Retrieved 15 June 2023.
- ↑ James, AL; Thorp, MA (1 March 2007). "Menière's disease". BMJ clinical evidence. 2007. PMID 19454061.
- ↑ Botta L, Mira E, Valli S, Zucca G, Perin P, Benvenuti C, Fossati A, Valli P (June 2001). "Effects of betahistine and of its metabolites on vestibular sensory organs". Acta Otorhinolaryngol Ital. 21 (3 Suppl 66): 24–30. PMID 11677836.
External links
| Identifiers: |
|---|