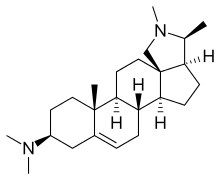
Conessine
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(3S,3aS,5aS,5bR,9S,11aR,11bS,13aR)-N,N,2,3,11a-Pentamethyl-2,3,3a,4,5,5a,5b,6,8,9,10,11,11a,11b,12,13-hexadecahydro-1H-naphtho[2′,1′:4,5]indeno[1,7a-c]pyrrol-9-amine | |
| Other names
Neriine; Roquessine; Wrightine; Conessinum; (3β)-N,N-Dimethyl-con-5-enin-3-amine | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.008.089 |
| MeSH | Conessine |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C24H40N2 |
| Molar mass | 356.598 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Conessine is a steroid alkaloid found in a number of plant species from the family Apocynaceae, including Holarrhena floribunda,[1] Holarrhena antidysenterica[2] and Funtumia elastica.[3] It acts as a histamine antagonist, selective for the H3 subtype (with an affinity of pKi = 8.27; Ki = ~5 nM).[4] It was also found to have long CNS clearance times, high blood-brain barrier penetration and high affinity for the adrenergic receptors.[5]
References
- ↑ Duez, P; Chamart, S; Lejoly, J; Hanocq, M; Zeba, B; Sawadogo, M; Guissou, P; Molle, L (1987). "Changes in conessine in stem bark of Holarrhena floribunda in Burkina Faso". Annales pharmaceutiques françaises. 45 (4): 307–13. PMID 3445993.
- ↑ Kumar, N; Singh, B; Bhandari, P; Gupta, A. P.; Kaul, V. K. (2007). "Steroidal alkaloids from Holarrhena antidysenterica (L.) WALL". Chemical & Pharmaceutical Bulletin. 55 (6): 912–4. doi:10.1248/cpb.55.912. PMID 17541193.
- ↑ Zirihi, G. N.; Grellier, P; Guédé-Guina, F; Bodo, B; Mambu, L (2005). "Isolation, characterization and antiplasmodial activity of steroidal alkaloids from Funtumia elastica (Preuss) Stapf". Bioorganic & Medicinal Chemistry Letters. 15 (10): 2637–40. doi:10.1016/j.bmcl.2005.03.021. PMID 15863333.
- ↑ Santora, V. J.; Covel, J. A.; Hayashi, R; Hofilena, B. J.; Ibarra, J. B.; Pulley, M. D.; Weinhouse, M. I.; Sengupta, D; Duffield, J. J.; Semple, G; Webb, R. R.; Sage, C; Ren, A; Pereira, G; Knudsen, J; Edwards, J. E.; Suarez, M; Frazer, J; Thomsen, W; Hauser, E; Whelan, K; Grottick, A. J. (2008). "A new family of H3 receptor antagonists based on the natural product Conessine". Bioorganic & Medicinal Chemistry Letters. 18 (4): 1490–4. doi:10.1016/j.bmcl.2007.12.059. PMID 18194865.
- ↑ Zhao, Chen; Sun, Minghua; Bennani, Youssef L.; Gopalakrishnan, Sujatha M.; Witte, David G.; Miller, Thomas R.; Krueger, Kathleen M.; Browman, Kaitlin E.; Thiffault, Christine; Wetter, Jill; Marsh, Kennan C.; Hancock, Arthur A.; Esbenshade, Timothy A.; Cowart, Marlon D. (2008). "The Alkaloid Conessine and Analogues as Potent Histamine H3Receptor Antagonists". Journal of Medicinal Chemistry. 51 (17): 5423–30. doi:10.1021/jm8003625. PMID 18683917.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.