Moxastine
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.020.626 |
| Chemical and physical data | |
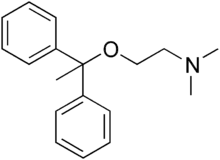
| Formula | C18H23NO |
| Molar mass | 269.388 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Moxastine (also known as mephenhydramine) is an antihistamine and anticholinergic.
It was developed in Czechoslovakia and sold in hydrochloride form as an antihistamine (Alfadryl).
It is, with 8-chlorotheophylline, a component of cocrystal/salt moxastine teoclate (mephenhydrinate) used as antiemetic (Theadryl; Kinedryl (with caffeine)).
References
See also
Histamine receptor modulators | |||||
|---|---|---|---|---|---|
| H1 |
| ||||
| H2 |
| ||||
| H3 |
| ||||
| H4 |
| ||||
See also: Receptor/signaling modulators • Monoamine metabolism modulators • Monoamine reuptake inhibitors | |||||
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.