Bilastine
 | |
| Clinical data | |
|---|---|
| Trade names | Ilaxten |
| Routes of administration | By mouth |
| Drug class | Antihistamine |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.260.016 |
| Chemical and physical data | |
| Formula | C28H37N3O3 |
| Molar mass | 463.622 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Bilastine, sold under the brand name Ilaxten among others, is a second-generation antihistamine medication which is used in the treatment of allergic rhinoconjunctivitis and urticaria (hives).[1]
It exerts its effect as a selective histamine H1 receptor antagonist,[2] and has an effectiveness similar to cetirizine, fexofenadine, and desloratadine.[3] It was developed in Spain by FAES Farma. It is sold under the brand name of Bilargo (Ziska Pharmaceuticals Ltd.) in Bangladesh.
Bilastine is approved in the European Union for the symptomatic treatment of allergic rhinoconjunctivitis and urticaria,[4] but it is not approved for any use in the United States.[5] Bilastine meets the current European Academy of Allergy and Clinical Immunology (EAACI) and Allergic Rhinitis and its Impact of Asthma (ARIA) criteria for medication used in the treatment of allergic rhinitis.[6]
Bilastine has been effective in the treatment of diseases of allergies, including rhinoconjuctivitis.[3] Additionally, bilastine has been shown to improve quality of life, and all nasal and eye symptoms related to allergic rhinitis.[6]
Medical uses
Allergic rhinoconjunctivitis
The clinical efficacy of bilastine in allergic rhinitis (AR) and urticaria has been assessed in 10 clinical assays in which over 4,600 patients were involved. All of them compared bilastine with placebo and another second generation antihistamine with confirmed efficacy (active comparator).
Allergic rhinitis
The studies on SAR were double-blind, placebo-controlled, parallel-group involving male and female patients over 12 year of age with symptomatic disease at the beginning of the study. Nasal symptoms (sneezing, rhinorrhea, nasal itching and congestion) were assessed both before treatment and during treatment period on a daily basis. Non nasal symptoms (itchy eye, watery eye, itchy ear and palate) were also assessed according to a 0–3 scale, so that the Total Symptoms Score (TSS) and other related parameters could clearly reflect daily evolution of SAR in each patient and treatment group. Parameters such as quality of life and discomfort were also assessed, and in the same way the type and frequency of AE, tolerability and general safety of treatment were registered. In this SAR studies the daily oral administration during 14 days of bilastine proves to have the same efficacy as the administration of cetirizine and desloratadine.[7]
Urticaria
A review article evaluated data from trials which detailed the efficacy of bilastine in skin models and urticaria to assess whether bilastine has an optimal profile for updosing in urticaria. The authors concluded that bilastine has an excellent profile for both efficacy and safety, although there is a need for controlled clinical trials to compare the efficacy of bilastine in a real-life updosing study in patients with urticaria, paying special attention to itch control.[8]
Dosage
It is taken by mouth and it is supposed to be swallowed. It shows a rapid onset of action (within 30–60 minutes).[3] It should be taken only by children older than 12 years and adults.[6]
Side effects
Toxicity of bilastine investigated in preclinical toxicology studies in mice, rats and dogs after oral and intravenous administration showed no mortality observed after oral administration of massive doses. After intravenous administration, LD50 (lethal dose for 50% of animals) values were 33 and 45–75 mg/kg in mice and rats, respectively. No signs of toxicity were observed in any organ after bilastine massive overdosing, either orally (in mice, rats and dogs), or intravenously (in rats and dogs) during 4 weeks. No effects on fertility, no teratogenic or mutagenic effects, and no apparent carcinogenic potential were seen in the studies carried out in rats, mice and rabbits.[9]
In clinical research, bilastine has proven to be well tolerated, with an adverse events profile similar to that of placebo in healthy volunteers, patients with AR and with chronic idiopathic urticaria. Although the tolerance profile of bilastine and levocetirizine or desloratadine were very similar,[10] bilastine was markedly better tolerated than cetirizine in a clinical assay in SAR, with fewer adverse events in the bilastine group. No anticholinergic adverse events were observed in the clinical trials with bilastine. No serious adverse events were reported during the research and there were no clinically significant changes in vital signs, electrocardiography (ECG) or laboratory tests. Pharmacokinetic/pharmacodynamic profiles and studies in special populations indicate that dose adjustment of bilastine is not necessary in elderly patients or in chronic liver disease or chronic kidney disease.
Cardiac safety
The clinical cardiac safety of bilastine has been assessed in all of the clinical trials performed so far (more than 3,500 patients treated with bilastine) and in a phase I study (Thorough QT/QTc study) designed according to the ICH E14 guidance and the most demanding requirements from the Food and Drug Administration (FDA). When electrocardiograms (ECG) data from all of the phase I studies are analysed, no significant alteration is appreciated in any of the parameters after administering bilastine at single doses (up to 11 times the therapeutic dose), nor at multiple doses (up to 10 times the therapeutic dose). Phase II and III studies on AR and urticaria (including the open-label extension phase of 12 months) do not reveal alterations in the ECG, nor significant prolongations of the QTc interval after administration of bilastine 20 mg.
The Thorough QT/QTc study was designed to assess the effect on the QT/QTc interval, both of the therapeutic dose (20 mg) and 100 mg of bilastine, but also the coadministration of the therapeutic dose with usual doses of ketoconazol (400 mg/day), a metabolism inhibitor and a P-gP dependent transport system. It was verified that bilastine 20 and 100 mg administered during 4 days, does not induce significant changes in the QT/QTc interval duration in any of the individuals.[11][12] Likewise, coadministration of bilastine 20 mg and ketoconazol 400 mg does not produce any significant prolongation of the QT/QTc interval attributable to bilastine.
Interactions
Preclinical data suggest the possibility of interactions between bilastine and drugs or food that are inhibitors or inducers of the P-glycoproteins. Coadministration of bilastine and grapefruit juice (a known P-glycoprotein-mediated drug transport activator) significantly reduced bilastine systemic exposure.[13] This interaction is due to the known effect of grapefruit flavonoids on intestinal transporter systems such as P-glycoproteins and organic anion transporting peptide (OATP).[14]
Pharmacology
Pharmacodynamics
Bilastine binds to guinea-pig cerebellar histamine H1-receptors (Ki=44 nM) and to human recombinant histamine H1-receptors (Ki=64 nM) with an affinity comparable to that of astemizole and diphenhydramine, and superior than that of cetirizine by three-fold and fexofenadine by five-fold (Corcóstegui). In different murine models, bilastine by oral route, antagonizes the effects of histamine in a dose-dependent manner, with potency similar to that of cetirizine and between 5.5 and 10 times greater than that of fexofenadine.[15]
Preclinical investigations demonstrate the affinity and specificity of bilastine for histamine H1-receptors compared with other histamine receptors subtypes and other 30 receptors from different amines. In vivo experimentation confirmed the antihistaminic and antiallergic activity, which was at least comparable to that of other second-generation H1-antihistamines such as cetirizine.
Pharmacokinetics
Absorption
Bilastine is most quickly absorbed with the absence of food, and reaches a mean peak plasma concentration of 220 ng/mL approximately 1 h after both single and multiple dosing.[16] Absorption is reduced by a high-fat breakfast or fruit juice, and the estimated global oral bioavailability is approximately 60%.[16] Bilastine has linear pharmacokinetics in the 2.5–220 mg dose range in healthy adult subjects without evidence of accumulation after 14 days of treatment.[16]
Distribution
Bilastine distribution has an apparent volume of distribution of 1.29 L/kg, and has an elimination half-life of 14.5 h and plasma protein binding of 84–90%.[17]
Metabolism
Bilastine is not significantly metabolized in humans and is largely eliminated unchanged both in urine and feces – a third and two thirds of the administered dose, respectively, according to a Phase I mass-balance study with radiolabeled bilastine.[18] Bilastine does not readily cross the blood brain barrier and is not metabolized by the liver.[16] Ninety six percent of the administered dose is eliminated within 24 hours.[16]
In relation to its antihistamine effect, oral doses of 20 mg daily of bilastine, measured as skin wheal-and-flare surface areas for 24 h, bilastine is capable of inhibiting 50% of the surface areas – throughout the whole administration interval.[16]
Chemistry
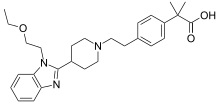
Bilastine, or 2-[4-[2-[4-[1-(2-ethoxyethyl) benzimidazol-2-yl] piperidin-1-yl] ethyl] phenyl]-2-methylpropionic acid, is a novel molecule with a molecular weight of 463.6 daltons and a chemical structure similar to piperidinyl-benzimidazole.[16] Bilastine can be therefore classified into the same chemical group as many of the new antihistamines on the market, although it is not structurally derived, nor is it a metabolite or enantiomer of any of them, but an original molecule designed with the intent of fulfilling all the requirements of a second-generation antihistamine.[16]
Research
Clinical studies using different dosages were done on histamine-induced wheal and flare reaction over a 24-h period, compared with a single 10-mg oral dose of cetirizine.[16] The results of this research indicated that bilastine was at least as efficient as cetirizine in reducing histamine-mediated effects in healthy volunteers. Remarkably, 20 and 50 mg of bilastine reduced the wheal and flare reaction significantly more quickly than cetirizine.[16]
References
- 1 2 "Ilaxten 20 mg tablets - Summary of Product Characteristics (SmPC)". (emc). Retrieved 16 June 2021.
- ↑ Corcóstegui R, Labeaga L, Innerárity A, Berisa A, Orjales A (2005). "Preclinical pharmacology of bilastine, a new selective histamine H1 receptor antagonist: Receptor selectivity and in vitro antihistaminic activity". Drugs in R&D. 6 (6): 371–84. doi:10.2165/00126839-200506060-00005. PMID 16274260. S2CID 23407135.
- 1 2 3 Jáuregui I, Bartra J, del Cuvillo A, Dávila I, Ferrer M, Montoro J, Mullol J, Sastre J, Valero A (2011). "Bilastine and quality of life". Journal of Investigational Allergology and Clinical Immunology. 21 Suppl 3: 16–23. PMID 22185046.
- ↑ Cumulative Nce introduction index, 1983–2010. Annual Reports in Medicinal Chemistry. Vol. 46. 2011. pp. 531–551. doi:10.1016/B978-0-12-386009-5.00035-7. ISBN 9780123860095.
- ↑ Bilastine Approval Status, drugs.com
- 1 2 3 Bousquet J, Ansótegui I, Canonica GW, Zuberbier T, Baena-Cagnani CE, Bachert C, Cruz AA, González SN, Kuna P, Morais-Almeida M, Mullol J, Ryan DP, Sánchez-Borges M, Valiente R, Church MK (2012). "Establishing the place in therapy of bilastine in the treatment of allergic rhinitis according to ARIA: evidence review". Current Medical Research and Opinion. 28 (1): 131–9. doi:10.1185/03007995.2011.648263. PMID 22149770. S2CID 8429174.
- ↑ Bachert C, Kuna P, Sanquer F, Ivan P, Dimitrov V, Gorina MM, van de Heyning P, Loureiro A (2009). "Comparison of the efficacy and safety of bilastine 20 mg vs desloratadine 5 mg in seasonal allergic rhinitis patients". Allergy. 64 (1): 158–65. doi:10.1111/j.1398-9995.2008.01813.x. PMID 19132976. S2CID 20109223.
- ↑ Church MK, Labeaga L (2017). "Bilastine: a new H1 -antihistamine with an optimal profile for updosing in urticaria". J Eur Acad Dermatol Venereol. 31 (9): 1447–1452. doi:10.1111/jdv.14305. PMID 28467671. S2CID 35712759.
- ↑ Horak F, Zieglmayer P, Zieglmayer R, Lemell P (2010). "The effects of bilastine compared with cetirizine, fexofenadine, and placebo on allergen-induced nasal and ocular symptoms in patients exposed to aeroallergen in the Vienna challenge chamber". Inflamm. Res. 59 (5): 391–398. doi:10.1007/s00011-009-0117-4. PMID 19943178. S2CID 30289994.
- ↑ Kuna P, Bachert C, Nowacki Z, van Cauwenberge P, Agache I, Fouquert L, Roger A, Sologuren A, Valiente R (2009). "Efficacy and safety of bilastine 20 mg compared with cetirizine 10 mg and placebo for the symptomatic treatment of seasonal allergic rhinitis: a randomized, double-blind, parallel-group study". Clin. Exp. Allergy. 39 (9): 1338–1347. doi:10.1111/j.1365-2222.2009.03257.x. PMID 19438584. S2CID 42461412.
- ↑ Tyl B, Kabbaj M, Azzam S, Sologuren A, Valiente R, Reinbolt E, Roupe K, Blanco N, Wheeler W (2012). "Lack of significant effect of bilastine administered at therapeutic and supratherapeutic doses and concomitantly with ketoconazole on ventricular repolarization: Results of a thorough QT study (TQTS) with QT-concentration analysis". Journal of Clinical Pharmacology. 52 (6): 893–903. doi:10.1177/0091270011407191. PMID 21642470. S2CID 11649589.
- ↑ Graff C, Struijk JJ, Kanters JK, Andersen MP, Toft E, Tyl B (2012). "Effects of bilastine on T-wave morphology and the QTc interval: A randomized, double-blind, placebo-controlled, thorough QTc study". Clinical Drug Investigation. 32 (5): 339–51. doi:10.2165/11599270-000000000-00000. PMID 22393898. S2CID 22766684.
- ↑ Bachert, C.; Kuna, P.; Zuberbier, T. (1 June 2010). "Bilastine in allergic rhinoconjunctivitis and urticaria". Allergy. 65: 1–13. doi:10.1111/j.1398-9995.2010.02404.x. S2CID 52228628.
- ↑ Bailey DG (2010). "Fruit juice inhibition of uptake transport: a new type of food-drug interaction". Br. J. Clin. Pharmacol. 70 (5): 645–655. doi:10.1111/j.1365-2125.2010.03722.x. PMC 2997304. PMID 21039758.
- ↑ Corcóstegui R, Labeaga L, Innerárity A, Berisa A, Orjales A (2005). "Preclinical pharmacology of bilastine, a new selective histamine H1 receptor antagonist. Receptor selectivity and in vitro antihistaminic activity". Drugs in R&D. 6 (6): 371–384. doi:10.2165/00126839-200506060-00005. PMID 16274260. S2CID 23407135.
- 1 2 3 4 5 6 7 8 9 10 Jáuregui I, García-Lirio E, Soriano AM, Gamboa PM, Antépara I (1 January 2012). "An overview of the novel H1-antihistamine bilastine in allergic rhinitis and urticaria". Expert Review of Clinical Immunology. 8 (1): 33–41. doi:10.1586/eci.11.87. PMID 22149338. S2CID 207209051.
- ↑ Jauregizar N, de la Fuente L, Lucero ML, Sologuren A, Leal N, Rodríguez M (1 August 2009). "Pharmacokinetic-Pharmacodynamic Modelling of the Antihistaminic (H1) Effect of Bilastine". Clinical Pharmacokinetics. 48 (8): 543–554. doi:10.2165/11317180-000000000-00000. PMID 19705924. S2CID 552051.
- ↑ "Human mass balance with [14 C]-bilastine following oral administration to healthy volunteers". Basic Clin. Pharmacol. Toxicol. 105. 2009.
External links
- "Bilastine". Drug Information Portal. U.S. National Library of Medicine.