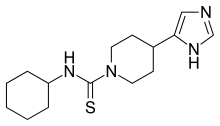
Thioperamide
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C15H24N4S |
| Molar mass | 292.45 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Thioperamide is a potent HRH4 antagonist and selective HRH3 antagonist capable of crossing the blood–brain barrier.[1] It was used by Jean-Charles Schwartz in his early experiments regarding the H3 receptor.[2] Thioperamide was found to be an antagonist of histamine autoreceptors, which negatively regulate the release of histamine, and enhances the activity of histaminergic neurons by blocking autoreceptors, leading to greater release of histamine.
Its action on H3 is thought to promote wakefulness and improve memory consolidation.
See also
References
- ↑ "Thioperamide". IUPHAR/BPS Guide to Pharmacology.
- ↑ Schwartz JC (June 2011). "The histamine H3 receptor: from discovery to clinical trials with pitolisant". British Journal of Pharmacology. 163 (4): 713–21. doi:10.1111/j.1476-5381.2011.01286.x. PMC 3111674. PMID 21615387.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.