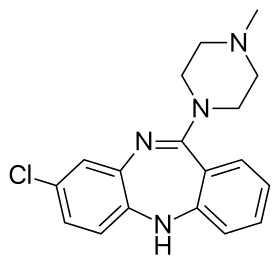
Clozapine
 | |
 | |
| Names | |
|---|---|
| Trade names | Clozaril, Leponex, Versacloz, others[1] |
IUPAC name
| |
| Clinical data | |
| Drug class | Atypical antipsychotic[2] |
| Main uses | Schizophrenia[2] |
| Side effects | Low white cells[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth, intramuscular injection |
| Defined daily dose | 300 mg (by injection or by mouth)[3] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a691001 |
| Legal | |
| License data | |
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | 60 to 70% |
| Metabolism | Liver, by several CYP isozymes |
| Elimination half-life | 4 to 26 hours (mean value 14.2 hours in steady state conditions) |
| Excretion | 80% in metabolized state: 30% biliary and 50% kidney |
| Chemical and physical data | |
| Formula | C18H19ClN4 |
| Molar mass | 326.83 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 183 °C (361 °F) |
| Solubility in water | 0.1889[4] mg/mL (20 °C) |
SMILES
| |
InChI
| |
Clozapine, sold under the brand name Clozaril among others,[1] is an atypical antipsychotic medication.[2] It is mainly used for schizophrenia that does not improve following the use of other antipsychotic medications.[2] In those with schizophrenia and schizoaffective disorder it may decrease the rate of suicidal behavior.[2] It is more effective than typical antipsychotics, particularly in those who are treatment-resistant.[5][6][7] It is used by mouth,[2] or by injection into a muscle.[8]
Clozapine is associated with a relatively high risk of low white cells (agranulocytosis), a condition of suppressed immunity which may result in death.[2] To decrease this risk, it is recommended that the white blood cell count be regularly monitored.[2] Other serious risks include seizures, inflammation of the heart, high blood sugar levels, constipation, and in older people with psychosis as a result of dementia, an increased risk of death.[2][9][10] Common side effects include drowsiness, increased saliva production, low blood pressure, blurred vision, and dizziness.[2] The potentially permanent movement disorder tardive dyskinesia occurs in about 5% of people.[9] Its mechanism of action is not entirely clear.[2]
Clozapine was first made in 1956, and sold commercially in 1972.[11][12] It was the first atypical antipsychotic.[13] It is on the World Health Organization's List of Essential Medicines.[14] It is available as a generic medication.[2] The wholesale cost in the developing world is between US$0.05 and US$2.10 per day as of 2014.[15]
Medical uses
Clozapine is an atypical antipsychotic drug primarily used in people who are unresponsive to or intolerant to other antipsychotics.[6] This means that they have failed to respond satisfactorily to at least two different antipsychotics.[16] It has been shown to be more effective in reducing symptoms of schizophrenia than typical antipsychotics, with more pronounced effects in those who have responded poorly to other medication.[5] It may also be used for the treatment of psychosis secondary to Parkinson's disease.[17]
Clozapine is usually given by mouth in tablet or liquid form,[2] however an unlicensed short-acting intramuscular injectable formulation is available. It is not a depot injection, and instead has a similar duration of action as clozapine by mouth. The injectable formulation may be used in highly agitated people with schizophrenia who consistently refuse clozapine by mouth, but are predicted to respond well to clozapine therapy, with the injection being administered with the intention of transitioning the person to oral clozapine.[8] The injectable form is reportedly difficult to use due to painful administration, higher doses requiring injection into multiple sites simultaneously, and even more stringent monitoring than oral clozapine (with the additional difficulty of withdrawing blood samples for testing from agitated individuals).[8]
In a 2013 study in a comparison of 15 antipsychotic drugs in effectiveness in treating schizophrenic symptoms, clozapine was ranked first and demonstrated very high effectiveness. 25% more effective than amisulpride (2nd), 33% more effective than olanzapine (3rd), and twice as effective as haloperidol, quetiapine, and aripiprazole.[5]
The effect of clozapine, however, is not (at least in the short term) reflected in measures of global functioning such as ability to leave the hospital and maintain an occupation. The relapse rate is lower and patient acceptability is better.[6] There is some evidence clozapine may reduce propensity for substance abuse in schizophrenic patients.[18]
It may be better than other antipsychotics in people with both schizophrenia and Parkinson's disease.[19]
Dosage
The defined daily dose is 300 mg by mouth or by injection.[3]
Side effects
Clozapine may cause side effects, some of which are serious and potentially fatal. Common side effects include constipation, bed-wetting, night-time drooling, muscle stiffness, sedation, tremors, orthostatic hypotension, hyperglycemia, and weight gain. The risk of developing extrapyramidal symptoms, such as tardive dyskinesia is below that of typical antipsychotics; this may be due to clozapine's anticholinergic effects. Extrapyramidal symptoms may subside somewhat after a person switches from another antipsychotic to clozapine.[20]
Clozapine carries five black box warnings, including warnings for agranulocytosis, central nervous system depression, leukopenia, neutropenia, seizure disorder, bone marrow suppression, dementia, hypotension, myocarditis, orthostatic hypotension (with or without syncope) and seizures.[21] Lowering of the seizure threshold may be dose related and slow initial titration of dose may decrease the risk for precipitating seizures. Slow titration of dosing may also decrease the risk for orthostatic hypotension and other adverse cardiovascular side effects.[22]
Many males have experienced cessation of ejaculation during orgasm as a side effect of clozapine, though this is not documented in official drug guides.[23]
However, many side-effects can be managed and may not warrant discontinuation.[24]
Agranulocytosis
Clozapine carries a black box warning for drug-induced agranulocytosis. Without monitoring, agranulocytosis occurs in about 1% of people who take clozapine during the first few months of treatment;[25] the risk of developing it is highest about three months into treatment, and decreases substantially thereafter, to less than 0.01% after one year.[26]
Clozapine-induced agranulocytosis can be transient.[27]
Rapid point-of-care tests may simplify the monitoring for agranulocytosis.[28]
Early findings suggest that the concurrent use of granulocyte colony-stimulating factor (GCSF) to maintain the neutrophil count whilst on rechallenge following neutropenia, is safe and effective. But, if agranulocytosis was later to occur there would be little if any therapeutic alternative which would ordinarily be GCSF.[29][30]
Cardiac toxicity
Myocarditis is a sometimes fatal side effect of clozapine, which usually develops within the first month of commencement.[31] First manifestations of illness are fever which may be accompanied by symptoms associated with upper respiratory tract, gastrointestinal or urinary tract infection. Typically C-reactive protein (CRP) increases with the onset of fever and rises in the cardiac enzyme, troponin, occur up to 5 days later. Monitoring guidelines advise checking CRP and troponin at baseline and weekly for the first 4 weeks after clozapine initiation and observing the patient for signs and symptoms of illness.[32] Signs of heart failure are less common and may develop with the rise in troponin. A recent case-control study found that the risk of clozapine-induced myocarditis is increased with increasing rate of clozapine dose titration, increasing age and concomitant sodium valproate.[33]
Gastrointestinal hypomotility
Another underrecognized and potentially life-threatening side effect spectrum is gastrointestinal hypomotility, which may manifest as severe constipation, fecal impaction, paralytic ileus, bowel obstruction, acute megacolon, ischemia or necrosis.[34] Colonic hypomotility has been shown to occur in up to 80% of people prescribed clozapine when gastrointestinal function is measured objectively using radiopaque markers.[35] Clozapine-induced gastrointestinal hypomotility currently has a higher mortality rate than the better known side effect of agranulocytosis.[36] A Cochrane review found little evidence to help guide decisions about the best treatment for gastrointestinal hypomotility caused by clozapine and other antipsychotic medication.[37] Monitoring bowel function and the preemptive use of laxatives for all clozapine-treated people has been shown to improve colonic transit times and reduce serious sequelae.[38]
Increased saliva
Hypersalivation, or the excessive production of saliva, is one of the most common side effects of clozapine (30-80%).[39] The saliva production is especially bothersome at night and first thing in the morning, as the immobility of sleep precludes the normal clearance of saliva by swallowing that occurs throughout the day.[39] While clozapine is a muscarinic antagonist at the M1, M2, M3, and M5 receptors, clozapine is a full agonist at the M4 subset. Because M4 is highly expressed in the salivary gland, its M4 agonist activity is thought to be responsible for hypersalivation.[40] Clozapine-induced hypersalivation is likely a dose-related phenomenon, and tends to be worse when first starting the medication.[39] Besides decreasing the dose or slowing the initial dose titration, other interventions that have shown some benefit include systemically-absorbed anticholinergic medications like diphenhydramine[39] and topical anticholinergic medications like ipratropium bromide.[41] Mild hypersalivation may be managed by sleeping with a towel over the pillow at night.[41]
Central nervous system
CNS side effects include drowsiness, vertigo, headache, tremor, syncope, sleep disturbances, nightmares, restlessness, akinesia, agitation, seizures, rigidity, akathisia, confusion, fatigue, insomnia, hyperkinesia, weakness, lethargy, ataxia, slurred speech, depression, myoclonic jerks, and anxiety. Rarely seen are delusions, hallucinations, delirium, amnesia, libido increase or decrease, paranoia and irritability, abnormal EEG, worsening of psychosis, paresthesia, status epilepticus, and obsessive compulsive symptoms. Similar to other antipsychotics clozapine rarely has been known to cause neuroleptic malignant syndrome.[42]
Urinary incontinence
Clozapine is linked to urinary incontinence,[43] though its appearance may be under-recognized.[44]
Withdrawal effects
Abrupt withdrawal may lead to cholinergic rebound effects, severe movement disorders as well as severe psychotic decompensation. It has been recommended that patients, families, and caregivers be made aware of the symptoms and risks of abrupt withdrawal of clozapine. When discontinuing clozapine, gradual dose reduction is recommended to reduce the intensity of withdrawal effects.[45][46]
Weight gain and diabetes
In addition to hyperglycemia, significant weight gain is frequently experienced by patients treated with clozapine.[47] Impaired glucose metabolism and obesity have been shown to be constituents of the metabolic syndrome and may increase the risk of cardiovascular disease. The data suggest that clozapine may be more likely to cause adverse metabolic effects than some of the other atypical antipsychotics.[48] A study has established that olanzapine and clozapine disturb the metabolism by making the body take preferentially its energy from fat (instead of privileging carbohydrates). Levels of carbohydrates remaining high, the body develops insulin resistance (causing diabetes).[49]
Overdose
Fatalities have been reported due to clozapine overdose, though overdoses > 4000 mg have been survived.[50]
Drug interactions
Fluvoxamine inhibits the metabolism of clozapine leading to significantly increased blood levels of clozapine.[51]
When carbamazepine is concurrently used with clozapine, it has been shown to decrease plasma levels of clozapine significantly thereby decreasing the beneficial effects of clozapine.[52][53] Patients should be monitored for "decreased therapeutic effects of clozapine if carbamazepine" is started or increased. If carbamazepine is discontinued or the dose of carbamazepine is decreased, therapeutic effects of clozapine should be monitored. The study recommends carbamazepine to not be used concurrently with clozapine due to increased risk of agranulocytosis.[54]
Published case reports have stated that the use of benzodiazepines and clozapine concomitantly can result in severe adverse reaction such as respiratory arrest, cardiac arrest and sudden death.[55]
Ciprofloxacin is an inhibitor of CYP1A2 and clozapine is a major CYP1A2 substrate. Randomized study reported elevation in clozapine concentration in schizophrenia subjects concurrently taking ciprofloxacin.[56] Thus, the prescribing information for clozapine recommends "reducing the dose of clozapine by one-third of original dose" when ciprofloxacin and other CYP1A2 inhibitors are added to therapy, but once ciprofloxacin is removed from therapy, it is recommended to return clozapine to original dose.[57]
Pharmacology
Pharmacodynamics
| Protein | CZP Ki (nM) | NDMC Ki (nM) |
|---|---|---|
| 5-HT1A | 123.7 | 13.9 |
| 5-HT1B | 519 | 406.8 |
| 5-HT1D | 1,356 | 476.2 |
| 5-HT2A | 5.35 | 10.9 |
| 5-HT2B | 8.37 | 2.8 |
| 5-HT2C | 9.44 | 11.9 |
| 5-HT3 | 241 | 272.2 |
| 5-HT5A | 3,857 | 350.6 |
| 5-HT6 | 13.49 | 11.6 |
| 5-HT7 | 17.95 | 60.1 |
| α1A | 1.62 | 104.8 |
| α1B | 7 | 85.2 |
| α2A | 37 | 137.6 |
| α2B | 26.5 | 95.1 |
| α2C | 6 | 117.7 |
| β1 | 5,000 | 6,239 |
| β2 | 1,650 | 4,725 |
| D1 | 266.25 | 14.3 |
| D2 | 157 | 101.4 |
| D3 | 269.08 | 193.5 |
| D4 | 26.36 | 63.94 |
| D5 | 255.33 | 283.6 |
| H1 | 1.13 | 3.4 |
| H2 | 153 | 345.1 |
| H3 | >10,000 | >10,000 |
| H4 | 665 | 1,028 |
| M1 | 6.17 | 67.6 |
| M2 | 36.67 | 414.5 |
| M3 | 19.25 | 95.7 |
| M4 | 15.33 | 169.9 |
| M5 | 15.5 | 35.4 |
| SERT | 1,624 | 316.6 |
| NET | 3,168 | 493.9 |
| DAT | >10,000 | >10,000 |
| The smaller the value, the more strongly the drug binds to the site. All data are for cloned human proteins.[58][59] | ||
Clozapine is classified as an atypical antipsychotic drug because it binds to serotonin as well as dopamine receptors.[60]
Clozapine is an antagonist at the 5-HT2A subunit of the serotonin receptor, putatively improving depression, anxiety, and the negative cognitive symptoms associated with schizophrenia.[61][62]
A direct interaction of clozapine with the GABAB receptor has also been shown.[63] GABAB receptor-deficient mice exhibit increased extracellular dopamine levels and altered locomotor behaviour equivalent to that in schizophrenia animal models.[64] GABAB receptor agonists and positive allosteric modulators reduce the locomotor changes in these models.[65]
Clozapine induces the release of glutamate and D-serine, an agonist at the glycine site of the NMDA receptor, from astrocytes,[66] and reduces the expression of astrocytic glutamate transporters. These are direct effects that are also present in astrocyte cell cultures not containing neurons. Clozapine prevents impaired NMDA receptor expression caused by NMDA receptor antagonists.[67]
Pharmacokinetics
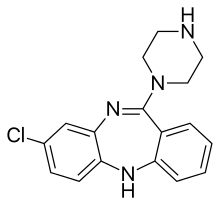
The absorption of clozapine is almost complete following oral administration, but the oral bioavailability is only 60 to 70% due to first-pass metabolism. The time to peak concentration after oral dosing is about 2.5 hours, and food does not appear to affect the bioavailability of clozapine. However, it was shown that co-administration of food decreases the rate of absorption.[68] The elimination half-life of clozapine is about 14 hours at steady state conditions (varying with daily dose).
Clozapine is extensively metabolized in the liver, via the cytochrome P450 system, to polar metabolites suitable for elimination in the urine and feces. The major metabolite, norclozapine (desmethyl-clozapine), is pharmacologically active. The cytochrome P450 isoenzyme 1A2 is primarily responsible for clozapine metabolism, but 2C, 2D6, 2E1 and 3A3/4 appear to play roles as well. Agents that induce (e.g., cigarette smoke) or inhibit (e.g., theophylline, ciprofloxacin, fluvoxamine) CYP1A2 may increase or decrease, respectively, the metabolism of clozapine. For example, the induction of metabolism caused by smoking means that smokers require up to double the dose of clozapine compared with non-smokers to achieve an equivalent plasma concentration.[69]
Clozapine and norclozapine (desmethyl-clozapine) plasma levels may also be monitored, though they show a significant degree of variation and are higher in women and increase with age.[70] Monitoring of plasma levels of clozapine and norclozapine has been shown to be useful in assessment of compliance, metabolic status, prevention of toxicity, and in dose optimisation.[69]
Chemistry
Clozapine is a dibenzodiazepine that is structurally related to loxapine. It is slightly soluble in water, soluble in acetone, and highly soluble in chloroform. Its solubility in water is 0.1889 mg/L (25 °C).[4] Its manufacturer, Novartis, claims a solubility of <0.01% in water (<100 mg/L).[71]
History
Clozapine was synthesized in 1956[11] by Wander AG, a Swiss pharmaceutical company, based on the chemical structure of the tricyclic antidepressant imipramine. The first test in humans in 1962 was considered a failure. Trials in Germany in 1965 and 1966 as well as a trial in Vienna in 1966 were successful. In 1967 Wander AG was acquired by Sandoz.[12] Further trials took place in 1972 when clozapine was released in Switzerland and Austria as Leponex. Two years later it was released in West Germany, and Finland in 1975. Early testing was performed in the United States around the same time.[12] In 1975, after reports of agranulocytosis leading to death in some clozapine-treated patients, clozapine was voluntarily withdrawn by the manufacturer.[72] Clozapine fell out of favor for more than a decade despite unclear reasons for the agranulocytosis which occurred in Finland, the rate of which was 20 times higher[73] than had been reported in any other country. However, when studies demonstrated that clozapine was more effective against treatment-resistant schizophrenia than other antipsychotics, the US Food and Drug Administration (FDA) and health authorities in most other countries approved its use only for treatment-resistant schizophrenia, and required Restricted Distribution, a Patient Registry and regular hematological monitoring to detect granulocytopenia, before agranulocytosis develops. In December 2002, clozapine was approved in the US for reducing the risk of suicide in schizophrenic or schizoaffective patients judged to be at chronic risk for suicidal behavior.[74] In 2005, the FDA approved criteria to allow reduced blood monitoring frequency.[75] In 2015, the individual manufacturer Patient Registries were consolidated by request of the FDA into a single shared Patient Registry Called The Clozapine REMS Registry.
Society and culture
Brand names
| A | Alemoxan, Azaleptine, Azaleptol |
| C | Cloment, Clonex, Clopin, Clopine, Clopsine, Cloril, Clorilex, Clozamed, Clozapex, Clozapin, Clozapina, Clozapinum, Clozapyl, Clozarem, Clozaril |
| D | Denzapine, Dicomex |
| E | Elcrit, Excloza |
| F | FazaClo, Froidir |
| I | Ihope |
| K | Klozapol |
| L | Lanolept, Lapenax, Leponex, Lodux, Lozapine, Lozatric, Luften |
| M | Medazepine, Mezapin |
| N | Nemea, Nirva |
| O | Ozadep, Ozapim |
| R | Refract, Refraxol |
| S | Sanosen, Schizonex, Sensipin, Sequax, Sicozapina, Sizoril, Syclop, Syzopin |
| T | Tanyl |
| U | Uspen |
| V | Versacloz |
| X | Xenopal |
| Z | Zaclo, Zapenia, Zapine, Zaponex, Zaporil, Ziproc, Zopin |
Cost
It is available as a generic medication.[2] The wholesale cost in the developing world is between 0.05 and US$2.10 per day as of 2014.[15] In the United Kingdom the injectable form is about 100 pounds per dose.[8]
See also
References
- 1 2 3 "Clozapine International Brands". Drugs.com. Archived from the original on 1 March 2017. Retrieved 28 February 2017.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 "Clozapine". The American Society of Health-System Pharmacists. Archived from the original on 8 December 2015. Retrieved 1 December 2015.
- 1 2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 3 July 2020. Retrieved 26 August 2020.
- 1 2 Hopfinger A, Esposito EX, Llinas A, Glen RC, Goodman JM (2009). "Findings of the Challenge To Predict Aqueous Solubility". Journal of Chemical Information and Modeling. 49 (1): 1–5. doi:10.1021/ci800436c. PMID 19117422.
- 1 2 3 Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, et al. (September 2013). "Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis". Lancet. 382 (9896): 951–62. doi:10.1016/S0140-6736(13)60733-3. PMID 23810019.
- 1 2 3 Essali A, Al-Haj Haasan N, Li C, Rathbone J (January 2009). "Clozapine versus typical neuroleptic medication for schizophrenia". The Cochrane Database of Systematic Reviews (1): CD000059. doi:10.1002/14651858.CD000059.pub2. PMC 7065592. PMID 19160174.
- ↑ Siskind D, McCartney L, Goldschlager R, Kisely S (November 2016). "Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis". The British Journal of Psychiatry. 209 (5): 385–392. doi:10.1192/bjp.bp.115.177261. PMID 27388573.
- 1 2 3 4 Hewitt, Jed; Haste, Jules (April 2017). "Protocol for the use of intramuscular (IM) clozapine injection" (PDF). Sussex Partnership NHS Foundation Trust. Archived (PDF) from the original on 29 August 2021. Retrieved 15 March 2019.
- 1 2 Hartling L, Abou-Setta AM, Dursun S, Mousavi SS, Pasichnyk D, Newton AS (October 2012). "Antipsychotics in adults with schizophrenia: comparative effectiveness of first-generation versus second-generation medications: a systematic review and meta-analysis". Annals of Internal Medicine. 157 (7): 498–511. doi:10.7326/0003-4819-157-7-201210020-00525. PMID 22893011.
- ↑ "Clozaril, Fazaclo ODT, Versacloz (clozapine): Drug Safety Communication - FDA Strengthens Warning That Untreated Constipation Can Lead to Serious Bowel Problems". FDA. 28 January 2020. Archived from the original on 24 February 2020. Retrieved 30 January 2020.
- 1 2 Haidary, HA; Padhy, RK (January 2019). "Clozapine". PMID 30571020.
{{cite journal}}: Cite journal requires|journal=(help) - 1 2 3 Crilly J (March 2007). "The history of clozapine and its emergence in the US market: a review and analysis". History of Psychiatry. 18 (1): 39–60. doi:10.1177/0957154X07070335. PMID 17580753.
- ↑ Li JJ, Corey JJ, eds. (2013). "Chapter 7: CNS Drugs". Drug discovery practices, processes, and perspectives. Hoboken, N.J.: John Wiley & Sons. p. 248. ISBN 9781118354469. Archived from the original on 10 September 2019. Retrieved 30 August 2017.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- 1 2 "Clozapine". International Drug Price Indicator Guide. Archived from the original on 22 January 2018. Retrieved 2 December 2015.
- ↑ Meltzer HY (1997). "Treatment-resistant schizophrenia--the role of clozapine". Current Medical Research and Opinion. 14 (1): 1–20. doi:10.1185/03007999709113338. PMID 9524789.
- ↑ British national formulary : BNF 74 (74 ed.). British Medical Association. 2017. p. 377. ISBN 978-0857112989.
- ↑ Lee M, Dickson RA, Campbell M, Oliphant J, Gretton H, Dalby JT (1998). "Clozapine and substance abuse in patients with schizophrenia". Canadian Journal of Psychiatry. 43 (8): 855–856. PMID 9806095.
- ↑ "How would you treat someone who has both schizophrenia and Parkinson's disease?". Archived from the original on 11 December 2013.
- ↑ "Clozapine". Archived from the original on 10 November 2013.
- ↑ "Clinical Pharmacology". www.clinicalpharmacology-ip.com. Archived from the original on 28 August 2021. Retrieved 10 November 2010.
- ↑ "Clozapine". Archived from the original on 10 November 2013.
- ↑ Baggaley M (April 2008). "Sexual dysfunction in schizophrenia: focus on recent evidence". Human Psychopharmacology. 23 (3): 201–209. doi:10.1002/hup.924. PMID 18338766.
- ↑ Nielsen J, Correll CU, Manu P, Kane JM (June 2013). "Termination of clozapine treatment due to medical reasons: when is it warranted and how can it be avoided?". The Journal of Clinical Psychiatry. 74 (6): 603–13. doi:10.4088/JCP.12r08064. PMID 23842012.
- ↑ Baldessarini, Ross J.; Frank I. Tarazi (2006). "Pharmacotherapy of Psychosis and Maa". In Laurence Brunton; John Lazo; Keith Parker (eds.). Goodman & Gilman's The Pharmacological Basis of Therapeutics (11th ed.). New York: McGraw-Hill. ISBN 978-0-07-142280-2. OCLC 150149056.
- ↑ Alvir JM, Lieberman JA, Safferman AZ, Schwimmer JL, Schaaf JA (July 1993). "Clozapine-induced agranulocytosis. Incidence and risk factors in the United States". The New England Journal of Medicine. 329 (3): 162–7. doi:10.1056/NEJM199307153290303. PMID 8515788. Free full text with registration Archived 2007-10-20 at the Wayback Machine
- ↑ Midbari Y, Ebert T, Kosov I, Kotler M, Weizman A, Ram A (October 2013). "Hematological and cardiometabolic safety of clozapine in the treatment of very early onset schizophrenia: a retrospective chart review". Journal of Child and Adolescent Psychopharmacology. 23 (8): 516–21. doi:10.1089/cap.2013.0050. PMID 24111981.
- ↑ Kalaria SN, Kelly DL (2019). "Development of point-of-care testing devices to improve clozapine prescribing habits and patient outcomes". Neuropsychiatr Dis Treat. 15: 2365–2370. doi:10.2147/NDT.S216803. PMC 6708436. PMID 31692521.
- ↑ Myles N, Myles H, Clark SR, Bird R, Siskind D (October 2017). "Use of granulocyte-colony stimulating factor to prevent recurrent clozapine-induced neutropenia on drug rechallenge: A systematic review of the literature and clinical recommendations". The Australian and New Zealand Journal of Psychiatry. 51 (10): 980–989. doi:10.1177/0004867417720516. PMID 28747065.
- ↑ Lally J, Malik S, Krivoy A, et al. (October 2017). "The Use of Granulocyte Colony-Stimulating Factor in Clozapine Rechallenge: A Systematic Review". Journal of Clinical Psychopharmacology. 37 (5): 600–604. doi:10.1097/JCP.0000000000000767. PMID 28817489. S2CID 41269943. Archived from the original on 6 July 2020. Retrieved 5 July 2020.
- ↑ Haas SJ, Hill R, Krum H, Liew D, Tonkin A, Demos L, Stephan K, McNeil J (2007). "Clozapine-associated myocarditis: a review of 116 cases of suspected myocarditis associated with the use of clozapine in Australia during 1993–2003". Drug Safety. 30 (1): 47–57. doi:10.2165/00002018-200730010-00005. PMID 17194170.
- ↑ Ronaldson KJ, Fitzgerald PB, Taylor AJ, Topliss DJ, McNeil JJ (June 2011). "A new monitoring protocol for clozapine-induced myocarditis based on an analysis of 75 cases and 94 controls". The Australian and New Zealand Journal of Psychiatry. 45 (6): 458–465. doi:10.3109/00048674.2011.572852. PMID 21524186.
- ↑ Ronaldson KJ, Fitzgerald PB, Taylor AJ, Topliss DJ, Wolfe R, McNeil JJ (November 2012). "Rapid clozapine dose titration and concomitant sodium valproate increase the risk of myocarditis with clozapine: a case-control study". Schizophrenia Research. 141 (2–3): 173–8. doi:10.1016/j.schres.2012.08.018. PMID 23010488.
- ↑ Palmer SE, McLean RM, Ellis PM, Harrison-Woolrych M (May 2008). "Life-threatening clozapine-induced gastrointestinal hypomotility: an analysis of 102 cases". The Journal of Clinical Psychiatry. 69 (5): 759–768. doi:10.4088/JCP.v69n0509. PMID 18452342.
- ↑ Every-Palmer S, Nowitz M, Stanley J, Grant E, Huthwaite M, Dunn H, Ellis, PM (March 2016). "Clozapine-treated patients have marked gastrointestinal hypomotility, the probable basis of life-threatening gastrointestinal complications: a cross sectional study". EBioMedicine. 5: 125–134. doi:10.1016/j.ebiom.2016.02.020. PMC 4816835. PMID 27077119.
- ↑ Cohen D, Bogers JP, van Dijk D, Bakker B, Schulte PF (2012). "Beyond white blood cell monitoring: screening in the initial phase of clozapine therapy". The Journal of Clinical Psychiatry. 73 (10): 1307–1312. doi:10.4088/JCP.11r06977. PMID 23140648.
- ↑ Every-Palmer S, Newton-Howes G, Clarke MJ (24 January 2017). "Pharmacological treatment for antipsychotic". The Cochrane Database of Systematic Reviews. 1 (1): CD011128. doi:10.1002/14651858.CD011128.pub2. PMC 6465073. PMID 28116777.
- ↑ Every-Palmer S, Ellis PM, Nowitz M, Stanley J, Grant E, Huthwaite M, Dunn H (January 2017). "The Porirua Protocol in the treatment of clozapine-induced gastrointestinal hypomotility and constipation: A pre- and post-treatment study". CNS Drugs. 31 (1): 75–85. doi:10.1007/s40263-016-0391-y. PMID 27826741.
- 1 2 3 4 Syed, R; Au, K; Cahill, C; Duggan, L; He, Y; Udu, V; Xia, J (16 July 2008). Syed, Rebecca (ed.). "Pharmacological interventions for clozapine-induced hypersalivation". The Cochrane Database of Systematic Reviews (3): CD005579. doi:10.1002/14651858.CD005579.pub2. PMC 4160791. PMID 18646130.
- ↑ "Treatment of Clozapine-Induced Sialorrhea". Archived from the original on 9 February 2012. Retrieved 8 February 2010.
- 1 2 Bird, Angela M; Smith, Tawny L; Walton, Amy E (3 May 2011). "Current Treatment Strategies for Clozapine-Induced Sialorrhea". Annals of Pharmacotherapy. 45 (5): 667–675. doi:10.1345/aph.1P761. PMID 21540404.
- ↑ "RxList Page Not Found". RxList. Archived from the original on 4 November 2007. Retrieved 4 June 2020.
- ↑ Raja M (July 2011). "Clozapine safety, 35 years later". Current Drug Safety. 6 (3): 164–184. doi:10.2174/157488611797579230. PMID 22122392.
- ↑ Barnes TR, Drake MJ, Paton C (January 2012). "Nocturnal enuresis with antipsychotic medication". The British Journal of Psychiatry. 200 (1): 7–9. doi:10.1192/bjp.bp.111.095737. PMID 22215862.
- ↑ Ahmed S, Chengappa KN, Naidu VR, Baker RW, Parepally H, Schooler NR (September 1998). "Clozapine withdrawal-emergent dystonias and dyskinesias: a case series". The Journal of Clinical Psychiatry. 59 (9): 472–7. doi:10.4088/JCP.v59n0906. PMID 9771818.
- ↑ Szafrański T, Gmurkowski K (1999). "[Clozapine withdrawal. A review]". Psychiatria Polska. 33 (1): 51–67. PMID 10786215.
- ↑ Wirshing DA, Wirshing WC, Kysar L, Berisford MA, Goldstein D, Pashdag J, Mintz J, Marder SR (June 1999). "Novel antipsychotics: comparison of weight gain liabilities". The Journal of Clinical Psychiatry. 60 (6): 358–63. doi:10.4088/JCP.v60n0602. PMID 10401912.
- ↑ Nasrallah HA (January 2008). "Atypical antipsychotic-induced metabolic side effects: insights from receptor-binding profiles". Molecular Psychiatry. 13 (1): 27–35. doi:10.1038/sj.mp.4002066. PMID 17848919.
- ↑ Albaugh VL, Vary TC, Ilkayeva O, Wenner BR, Maresca KP, Joyal JL, Breazeale S, Elich TD, Lang CH, Lynch CJ (January 2012). "Atypical antipsychotics rapidly and inappropriately switch peripheral fuel utilization to lipids, impairing metabolic flexibility in rodents". Schizophrenia Bulletin. 38 (1): 153–166. doi:10.1093/schbul/sbq053. PMC 3245588. PMID 20494946.
- ↑ Keck, PE Jr.; McElroy, SL (2002). "Clinical pharmacodynamics and pharmacokinetics of antimanic and mood-stabilizing medications". J Clin Psychiatry. 63 (Suppl 4): 3–11. PMID 11913673.
- ↑ Sproule BA, Naranjo CA, Brenmer KE, Hassan PC (December 1997). "Selective serotonin reuptake inhibitors and CNS drug interactions. A critical review of the evidence". Clinical Pharmacokinetics. 33 (6): 454–71. doi:10.2165/00003088-199733060-00004. PMID 9435993.
- ↑ Tiihonen J, Vartiainen H, Hakola P (January 1995). "Carbamazepine-induced changes in plasma levels of neuroleptics". Pharmacopsychiatry. 28 (1): 26–8. doi:10.1055/s-2007-979584. PMID 7746842.
- ↑ Besag FM, Berry D (2006). "Interactions between antiepileptic and antipsychotic drugs". Drug Safety. 29 (2): 95–118. doi:10.2165/00002018-200629020-00001. PMID 16454538.
- ↑ Jerling M, Lindström L, Bondesson U, Bertilsson L (August 1994). "Fluvoxamine inhibition and carbamazepine induction of the metabolism of clozapine: evidence from a therapeutic drug monitoring service". Therapeutic Drug Monitoring. 16 (4): 368–74. doi:10.1097/00007691-199408000-00006. PMID 7974626.
- ↑ Bitter R, Demler TL, Opler L (September 2008). "Safety evaluation of the concomitant use of clozapine and benzodiazepines: a retrospective, cross-sectional chart review". Journal of Psychiatric Practice. 14 (5): 265–70. doi:10.1097/01.pra.0000336753.11943.7c. PMID 18832957.
- ↑ Raaska K, Neuvonen PJ (November 2000). "Ciprofloxacin increases serum clozapine and N-desmethylclozapine: a study in patients with schizophrenia". European Journal of Clinical Pharmacology. 56 (8): 585–9. doi:10.1007/s002280000192. PMID 11151749.
- ↑ Prescribing information. Clozaril (clozapine). East Hanover, NJ: Novartis Pharmaceuticals Corporation, September 2014.
- 1 2 Roth, BL; Driscol, J. "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Archived from the original on 28 August 2021. Retrieved 14 August 2017.
- 1 2 Roth, BL; Driscol, J. "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Archived from the original on 28 August 2021. Retrieved 14 August 2017.
- ↑ Naheed M, Green B (2001). "Focus on clozapine". Current Medical Research and Opinion. 17 (3): 223–9. doi:10.1185/0300799039117069. PMID 11900316.
- ↑ Robinson DS (2007). "CNS Receptor Partial Agonists: A New Approach to Drug Discovery". Primary Psychiatry. 14 (8): 22–24. Archived from the original on 18 January 2012.
- ↑ "clozapine | C18H19ClN4 – PubChem". pubchem.ncbi.nlm.nih.gov. Archived from the original on 24 December 2013. Retrieved 16 July 2017.
- ↑ Wu Y, Blichowski M, Daskalakis ZJ, Wu Z, Liu CC, Cortez MA, Snead OC (September 2011). "Evidence that clozapine directly interacts on the GABAB receptor". NeuroReport. 22 (13): 637–41. doi:10.1097/WNR.0b013e328349739b. PMID 21753741.
- ↑ Vacher CM, Gassmann M, Desrayaud S, Challet E, Bradaia A, Hoyer D, Waldmeier P, Kaupmann K, Pévet P, Bettler B (May 2006). "Hyperdopaminergia and altered locomotor activity in GABAB1-deficient mice". Journal of Neurochemistry. 97 (4): 979–91. doi:10.1111/j.1471-4159.2006.03806.x. PMID 16606363.
- ↑ Wierońska JM, Kusek M, Tokarski K, Wabno J, Froestl W, Pilc A (July 2011). "The GABA B receptor agonist CGP44532 and the positive modulator GS39783 reverse some behavioural changes related to positive syndromes of psychosis in mice". British Journal of Pharmacology. 163 (5): 1034–47. doi:10.1111/j.1476-5381.2011.01301.x. PMC 3130949. PMID 21371011.
- ↑ Tanahashi S, Yamamura S, Nakagawa M, Motomura E, Okada M (March 2012). "Clozapine, but not haloperidol, enhances glial D-serine and L-glutamate release in rat frontal cortex and primary cultured astrocytes". British Journal of Pharmacology. 165 (5): 1543–55. doi:10.1111/j.1476-5381.2011.01638.x. PMC 3372736. PMID 21880034.
- ↑ Xi D, Li YC, Snyder MA, Gao RY, Adelman AE, Zhang W, Shumsky JS, Gao WJ (May 2011). "Group II metabotropic glutamate receptor agonist ameliorates MK801-induced dysfunction of NMDA receptors via the Akt/GSK-3β pathway in adult rat prefrontal cortex". Neuropsychopharmacology. 36 (6): 1260–74. doi:10.1038/npp.2011.12. PMC 3079418. PMID 21326193.
- ↑ DiSanto, Anthony R.; Golden, Gil (1 August 2009). "Effect of Food on the Pharmacokinetics of Clozapine Orally Disintegrating Tablet 12.5 mg". Clinical Drug Investigation. 29 (8): 539–549. doi:10.2165/00044011-200929080-00004. ISSN 1179-1918. PMID 19591515.
- 1 2 Rostami-Hodjegan A, Amin AM, Spencer EP, Lennard MS, Tucker GT, Flanagan RJ (February 2004). "Influence of dose, cigarette smoking, age, sex, and metabolic activity on plasma clozapine concentrations: a predictive model and nomograms to aid clozapine dose adjustment and to assess compliance in individual patients". Journal of Clinical Psychopharmacology. 24 (1): 70–8. doi:10.1097/01.jcp.0000106221.36344.4d. PMID 14709950.
- ↑ Lane HY, Chang YC, Chang WH, Lin SK, Tseng YT, Jann MW (January 1999). "Effects of gender and age on plasma levels of clozapine and its metabolites: analyzed by critical statistics". The Journal of Clinical Psychiatry. 60 (1): 36–40. doi:10.4088/JCP.v60n0108. PMID 10074876.
- ↑ Novartis Pharmaceuticals (April 2006). "Prescribing Information" (PDF). Novartis Pharmaceuticals. p. 36. Archived from the original on 23 October 2008. Retrieved 29 June 2007.
- ↑ Healy, David (2004). The Creation of Psychopharmacology. Cambridge: Harvard University Press. pp. 238–42. ISBN 978-0-674-01599-9. Archived from the original on 8 September 2017. Retrieved 4 June 2020.
- ↑ Griffith RW, Saameli K (October 1975). "Letter: Clozapine and agranulocytosis". Lancet. 2 (7936): 657. doi:10.1016/s0140-6736(75)90135-x. PMID 52022.
- ↑ "Supplemental NDA Approval Letter for Clozaril, NDA 19-758 / S-047" (PDF). United States Food and Drug Administration. 18 December 2002. Archived from the original (PDF) on 8 November 2013. Retrieved 23 November 2012.
- ↑ "Letter to Novartis Pharmaceuticals Corporation" (PDF). Archived (PDF) from the original on 11 May 2011. Retrieved 20 September 2009.
Further reading
- Benkert, Otto; Hippius, Hanns. Kompendium der Psychiatrischen Pharmakotherapie (in German) (4th ed.). Springer Verlag.
{{cite book}}: CS1 maint: unrecognized language (link) - Bandelow B, Bleich S, Kropp S. Handbuch Psychopharmaka (in German) (2nd ed.). Hogrefe.
{{cite book}}: CS1 maint: unrecognized language (link) - Crilly J (March 2007). "The history of clozapine and its emergence in the US market: a review and analysis". History of Psychiatry. 18 (1): 39–60. doi:10.1177/0957154X07070335. PMID 17580753.
- Dean L (2016). "Clozapine Therapy and CYP2D6, CYP1A2, and CYP3A4 Genotypes". In Pratt VM, McLeod HL, Rubinstein WS, et al. (eds.). Medical Genetics Summaries. National Center for Biotechnology Information (NCBI). PMID 28520368. Bookshelf ID: NBK367795. Archived from the original on 26 October 2020. Retrieved 5 February 2020.
External links
| External sites: |
|
|---|---|
| Identifiers: |