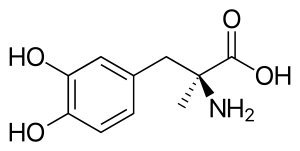
Methyldopa
 | |
 | |
| Names | |
|---|---|
| Trade names | Aldomet, Aldoril, Dopamet, others |
| Other names | L-α-Methyl-3,4-dihydroxyphenylalanine |
IUPAC name
| |
| Clinical data | |
| Drug class | Alpha-2 adrenergic receptor agonist[1] |
| Main uses | High blood pressure in pregnancy[2] |
| Side effects | Sleepiness[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | by mouth, IV |
| Onset of action | 4 to 6 hrs[1] |
| Duration of action | 10 to 48 hrs[1] |
| Defined daily dose | 1 to 2 gram[3] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682242 |
| Legal | |
| License data |
|
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | approximately 50% |
| Metabolism | Liver |
| Elimination half-life | 2 hours[4] |
| Excretion | Kidney for metabolites |
| Chemical and physical data | |
| Formula | C10H13NO4 |
| Molar mass | 211.217 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Methyldopa, sold under the brand name Aldomet among others, is a medication used for high blood pressure.[1] It is one of the preferred treatments for high blood pressure in pregnancy.[1] For other types of high blood pressure including very high blood pressure resulting in symptoms other medications are typically preferred.[1] It can be given by mouth or injection into a vein.[1] Onset of effects is around 5 hours and they last about a day.[1]
Common side effects include sleepiness.[1] More severe side effects include red blood cell breakdown, liver problems, and allergic reactions.[1] Methyldopa is in the alpha-2 adrenergic receptor agonist family of medication.[1] It works by stimulating the brain to decrease the activity of the sympathetic nervous system.[1]
Methyldopa was discovered in 1960.[5] It is on the World Health Organization's List of Essential Medicines.[6] The wholesale cost in the developing world is about US$4.31–9.48 per month.[7] In the United States it costs less than $25 per month.[8]
Medical uses
Methyldopa is used for high blood pressure including pregnancy-induced hypertension such as pre-eclampsia.
Dosage
The defined daily dose is 1 gram for the levorotatory version and 2 grams for the racemic version.[3] This is generally started at 250 mg two to three times a day for the first two days and than increased every few days by 250 mg until the typical long term dose of 1.5 grams is reached.[2] The maximum dose is 3 grams.[2]
Side effects
Methyldopa is capable of inducing a number of adverse side effects, which range from mild to severe. Nevertheless, they are generally mild when the dose is less than 1 gram per day.[9] Side effects may include:
- Psychological
- Depression or even suicidal ideation, as well as nightmares
- Apathy or anhedonia, as well as dysphoria
- Anxiety, especially of the social anxiety variant
- Decreased alertness, awareness, and wakefulness
- Impaired attention, focus, and concentration
- Decreased desire, drive, and motivation
- Fatigue or lethargy or malaise or lassitude
- Sedation or drowsiness or somnolence or sleepiness
- Agitation or restlessness
- Cognitive and memory impairment
- Derealization or depersonalization, as well as mild psychosis
- Sexual dysfunction including impaired libido, desire, and drive
- Physiological
- Dizziness, lightheadedness, or vertigo
- Miosis or pupil constriction
- Xerostomia or dry mouth
- Gastrointestinal disturbances such as diarrhea or constipation
- Headache or migraine
- Myalgia or muscle aches, arthralgia or joint pain, or paresthesia ("pins and needles")
- Restless legs syndrome (RLS)
- Parkinsonian symptoms such as muscle tremors, rigidity, hypokinesia, or balance or postural instability
- Akathisia, ataxia, dyskinesia as well as even tardive dyskinesia, or dystonia
- Bell's palsy or facial paralysis
- Sexual dysfunction consisting of impaired erectile dysfunction or anorgasmia
- Hyperprolactinemia or excess prolactin, gynecomastia/breast enlargement in males, or amenorrhoea or absence of menstrual cycles in females
- Bradycardia or decreased heart rate
- Hypotension or decreased blood pressure (though this may also be considered a therapeutic benefit)
- Orthostatic hypotension (also known as postural hypotension)
- Hepatitis, hepatotoxicity, or liver dysfunction or damage
- Pancreatitis or inflammation of the pancreas
- Warm autoimmune hemolytic anemia or deficiency in red blood cells (RBCs)
- Myelotoxicity or bone marrow suppression, potentially leading to thrombocytopenia or blood platelet deficiency or leukopenia or white blood cell (WBC) deficiency
- Hypersensitivity such as lupus erythematosus, myocarditis, or pericarditis
- Lichenoid reactions such as skin lesions or rashes
- Pallor
Rebound/withdrawal
Rebound hypertension via withdrawal on account of tolerance upon the abrupt discontinuation of methyldopa has been reported.[10]
Mechanism of action
The mechanism of action of methyldopa is not fully clear. Although it is a centrally acting sympathomimetic, it does not block reuptake or transporters. It may reduce the dopaminergic and serotonergic transmission in the peripheral nervous system and it indirectly affects norepinephrine (noradrenaline) synthesis.
The S-enantiomer of methyldopa is a competitive inhibitor of the enzyme aromatic L-amino acid decarboxylase (LAAD), which converts L-DOPA into dopamine. L-DOPA can cross the blood brain barrier and thus methyldopa may have similar effects. LAAD converts it into alpha-methyldopamine, a false prescursor to norepinephrine, which in turn reduces synthesis of norepinephrine in the vesicles. Dopamine beta hydroxylase (DBH) converts alpha-methyldopamine into alpha-methylnorepinephrine, which is an agonist of the presynaptic α2-adrenergic receptor causing inhibition of neurotransmitter release.
Pharmacokinetics
Methyldopa exhibits variable absorption from the gastrointestinal tract. It is metabolized in the liver and intestines and is excreted in urine.
History
When methyldopa was first introduced, it was the mainstay of antihypertensive treatment, but its use has declined on account of relatively severe adverse side effects, with increased use of other safer and more tolerable agents such as alpha blockers, beta blockers, and calcium channel blockers. Additionally, it has yet to be associated with reducing adverse cardiovascular events including myocardial infarction and stroke, or overall all-cause mortality reduction in clinical trials.[11] Nonetheless, one of methyldopa's still current indications is in the management of pregnancy-induced hypertension (PIH), as it is relatively safe in pregnancy compared to many other antihypertensives which may affect the fetus.
See also
- Difluoromethyldopa
- D-DOPA (dextrodopa)
- L-DOPA (levodopa; trade names Sinemet, Pharmacopa, Atamet, Stalevo, Madopar, Prolopa, etc.)
- L-DOPS (droxidopa)
- Dopamine (Intropan, Inovan, Revivan, Rivimine, Dopastat, Dynatra, etc.)
- Norepinephrine (noradrenaline; Levophed, etc.)
- Epinephrine (adrenaline; Adrenalin, EpiPed, Twinject, etc.)
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 "Methyldopa". The American Society of Health-System Pharmacists. Archived from the original on 21 December 2016. Retrieved 8 December 2016.
- 1 2 3 "METHYLDOPA oral - Essential drugs". medicalguidelines.msf.org. Archived from the original on 29 August 2021. Retrieved 31 August 2020.
- 1 2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 17 May 2021. Retrieved 31 August 2020.
- ↑ Benowitz, Neal L. (2020). "11. Antihypertensive agents". In Katzung, Bertram G.; Trevor, Anthony J. (eds.). Basic and Clinical Pharmacology (15th ed.). New York: McGraw-Hill. p. 183. ISBN 978-1-260-45231-0. Archived from the original on 2021-10-10. Retrieved 2021-12-05.
- ↑ Walker RS (2012). Trends and Changes in Drug Research and Development. Springer Science & Business Media. p. 109. ISBN 9789400926592. Archived from the original on 2016-09-14.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ "Methyldopa". International Drug Price Indicator Guide. Archived from the original on 27 August 2019. Retrieved 8 December 2016.
- ↑ Hamilton, Richart (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 140. ISBN 9781284057560.
- ↑ British National Formulary 56. September 2008. pp. 95–96. ISBN 978-0-85369-778-7.
- ↑ Methyldopa (PIM 342) Archived 2008-03-13 at the Wayback Machine
- ↑ Mah GT, Tejani AM, Musini VM (October 2009). "Methyldopa for primary hypertension". The Cochrane Database of Systematic Reviews (4): CD003893. doi:10.1002/14651858.CD003893.pub3. PMC 7154320. PMID 19821316.
External links
| Identifiers: |
|---|