DMMDA
 | |
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| ChemSpider |
|
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C12H19NO4 |
| Molar mass | 241.287 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
2,5-Dimethoxy-3,4-methylenedioxyamphetamine (DMMDA) is a psychedelic drug of the phenethylamine and amphetamine chemical classes.[1] It was first synthesized by Alexander Shulgin and was described in his book PiHKAL.[1] Shulgin listed the dosage as 30–75 mg and the duration as 6–8 hours.[1] He reported DMMDA as producing LSD-like images, mydriasis, ataxia, and time dilation.[1]
Synthesis
According to Alexander Shulgin, DMMDA can be synthesized from apiole.[1] Apiole is added to solution of potassium hydroxide and ethanol and the solution is held at a steam bath turning the apiole into isoapiole.[1] The isoapiole is then nitrated by adding it to a stirred solution of acetone and pyridine at ice-bath temperatures and treating the solution with tetranitromethane to form 1-(2,3-dimethoxy-3,4-methylenedioxyphenyl)-2-nitropropene.[1] The 1-(2,3-dimethoxy-3,4-methylenedioxyphenyl)-2-nitropropene is reduced to freebase DMMDA by adding it to a well-stirred and refluxing suspension of diethylether and lithium aluminum hydride under an inert atmosphere.[1] The freebase DMMDA converted into its hydrocloride salt.[1]
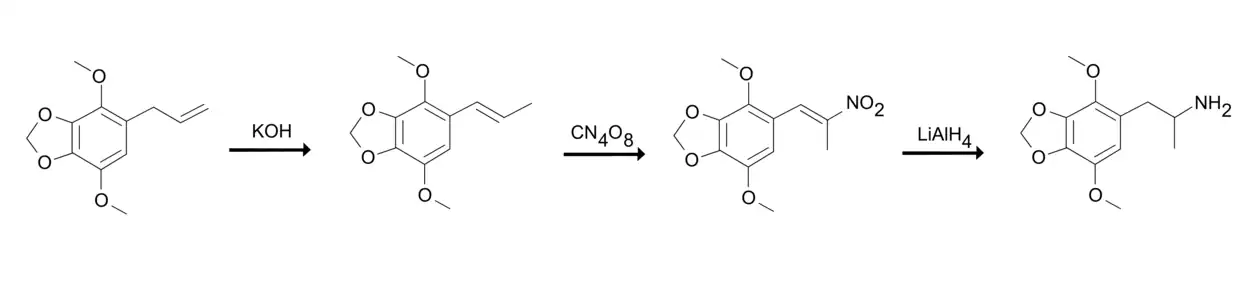 Alexander Shulgin's synthesis of DMMDA.
Alexander Shulgin's synthesis of DMMDA.
References
Empathogens/entactogens | |
|---|---|
| Phenylalkyl- amines (other than cathinones) |
|
| Cyclized phenyl- alkylamines | |
| Cathinones |
|
| Tryptamines | |
| Chemical classes | |
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|
Serotonin receptor modulators | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||