4C-T-2
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider |
|
| ChEMBL | |
| Chemical and physical data | |
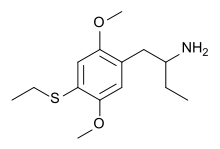
| Formula | C14H23NO2S |
| Molar mass | 269.40 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
2,5-Dimethoxy-4-ethylthio-α-ethylphenethylamine (4C-T-2) is a synthetic drug of the phenethylamine chemical class. It is the α-ethylated analogue of 2C-T-2.
Pharmacology
Binding profile
4C-T-2 has affinity (Ki) for the 5-HT1A (5,339 nM), 5-HT1E (9,879 nM), 5-HT2A (274.1 nM), 5-HT2B (58.1 nM), 5-HT2C (468.6 nM), 5-HT5A (1,587 nM), 5-HT7 (3,829), D3 (1,273 nM), β2-adrenergic (124.9 nM), I1 (946.5 nM), and σ1 (514 nM) receptors.[1] The activity of 4C-T-2 at these sites has not been assayed, with the exception of the 5-HT2A and 5-HT2C receptors where it acts as a partial agonist.[1]
See also
References
- 1 2 Ray TS (February 2010). "Psychedelics and the human receptorome". PLOS ONE. 5 (2): e9019. Bibcode:2010PLoSO...5.9019R. doi:10.1371/journal.pone.0009019. PMC 2814854. PMID 20126400.
| D1-like |
| ||||||
|---|---|---|---|---|---|---|---|
| D2-like |
| ||||||
| |||||||
Serotonin receptor modulators | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
Sigma receptor modulators | |
|---|---|
| σ1 |
|
| σ2 |
|
| Unsorted |
|
See also: Receptor/signaling modulators | |
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.