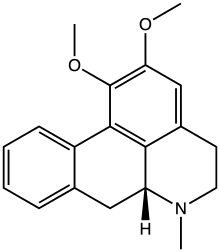
Nuciferine
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(6aR)-1,2-Dimethoxy-6-methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]quinoline | |
| Other names
(R)-1,2-Dimethoxyaporphine | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C19H21NO2 |
| Molar mass | 295.376 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Nuciferine is an alkaloid found within the plants Nymphaea caerulea and Nelumbo nucifera.
Preliminary psychopharmacological research in 1978 was unable to conclusively determine the compound's classification in regards to dopamine-receptor activity.[1] On one hand, investigative studies found evidence of behavior traditionally associated with dopamine-receptor stimulation: stereotypy, increase in spontaneous motor activity, inhibition of conditioned avoidance response, and an increase in pain sensitivity resulting in an inhibition of morphine analgesia.[1] On the other hand, these early investigative studies also found evidence of behavior traditionally associated with dopamine-receptor blockade: decrease of spontaneous motor activity, chills, catalepsy, trance-like states of consciousness.[1]
Nuciferine may also potentiate morphine analgesia. The median lethal dose in mice is 289 mg/kg. It is structurally related to apomorphine.[2][3]
Nuciferine has been reported to have various anti-inflammatory effects. Specific tests found nuciferine to reduce inflammation on lipopolysaccharide (LPS)-stimulated BV2 microglia cells. Researchers proposed that Nuciferine may have activated PPAR-y pathways to inhibit inflammation. More research into these pathways is necessary for a conclusive model for the anti-inflammatory response seen in laboratory studies.[4]
Nuciferine is a potential treatment for liver disease in Type-2 diabetic patients. In diabetic animal models with a high-fat diet, Nuciferine reversed liver damage. The underlying mechanism is unclear. The method of administration was a simple food additive. Culinary methods of preparing lotus flower (a botanical source of naturally-occurring Nuciferine) make this a potentially viable preventive method to prevent liver damage with diet.[5]
Nuciferine's ability to penetrate the blood brain barrier makes it an interesting candidate for treating diseases of the brain. Various anti-tumor and anti-viral pharmacological properties have been investigated. Nuciferine was shown to inhibit the progression of glioblastoma cancer cells by suppressing the SOX2-AKT/STAT3 signaling pathway in animal models. Targeting SOX-2 pathways in human models with Nuciferine may offer a novel therapeutic approach for cancer treatment.[6]
Nuciferine has shown efficacy as a treatment for premature ejaculation and erectile dysfunction.[7]
See also
- Apomorphine
- Aporphine
- Glaucine
- Bulbocapnine
- Nantenine
- Pukateine
- Stepholidine
- Tetrahydropalmatine
References
- 1 2 3 Bhattacharya, S. K.; Bose, R.; Ghosh, P.; Tripathi, V. J.; Ray, A. B.; Dasgupta, B. (1978-09-15). "Psychopharmacological studies on (--)-nuciferine and its Hofmann degradation product atherosperminine". Psychopharmacology. 59 (1): 29–33. doi:10.1007/bf00428026. ISSN 0033-3158. PMID 100809. S2CID 11847319.
- ↑ Bhattacharya SK, Bose R, Ghosh P, Tripathi VJ, Ray AB, Dasgupta B (Sep 1978). "Psychopharmacological studies on (—)-nuciferine and its Hofmann degradation product atherosperminine". Psychopharmacology. 59 (1): 29–33. doi:10.1007/BF00428026. PMID 100809. S2CID 11847319.
- ↑ Spess, David L. Errors in Alkaloids of Nelumbo and Nymphaea species, 2011, academia.edu
- ↑ Zhang, Lina; Gao, Jinghua; Tang, Peng; Chong, Li; Liu, Yue; Liu, Peng; Zhang, Xin; Chen, Li; Hou, Chen (October 2018). "Nuciferine inhibits LPS-induced inflammatory response in BV2 cells by activating PPAR-γ". International Immunopharmacology. 63: 9–13. doi:10.1016/j.intimp.2018.07.015. ISSN 1878-1705. PMID 30056259. S2CID 51894703.
- ↑ Zhang, Chao; Deng, Jianjun; Liu, Dan; Tuo, Xingxia; Xiao, Lei; Lai, Baochang; Yao, Qinyu; Liu, Jia; Yang, Haixia; Wang, Nanping (November 2018). "Nuciferine ameliorates hepatic steatosis in high-fat diet/streptozocin-induced diabetic mice through a PPARα/PPARγ coactivator-1α pathway". British Journal of Pharmacology. 175 (22): 4218–4228. doi:10.1111/bph.14482. ISSN 1476-5381. PMC 6193881. PMID 30129056.
- ↑ Li, Zizhuo; Chen, Yaodong; An, Tingting; Liu, Pengfei; Zhu, Jiyuan; Yang, Haichao; Zhang, Wei; Dong, Tianxiu; Jiang, Jian; Zhang, Yu; Jiang, Maitao (2019-03-29). "Nuciferine inhibits the progression of glioblastoma by suppressing the SOX2-AKT/STAT3-Slug signaling pathway". Journal of Experimental & Clinical Cancer Research. 38 (1): 139. doi:10.1186/s13046-019-1134-y. ISSN 1756-9966. PMC 6440136. PMID 30922391.
- ↑ Cai, Tommaso; Cocci, Andrea; Cito, Gianmartino; Giammusso, Bruno; Zucchi, Alessandro; Chiancone, Francesco; Carrino, Maurizio; Mastroeni, Francesco; Comerci, Francesco; Franco, Girgio; Palmieri, Alessandro (2018-03-31). "The role of diallyl thiosulfinate associated with nuciferine and diosgenin in the treatment of premature ejaculation: A pilot study". Archivio Italiano di Urologia, Andrologia. 90 (1): 59–64. doi:10.4081/aiua.2018.1.59. ISSN 1124-3562. PMID 29633800.