Alizapride
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral, IM, IV |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 3 hours |
| Excretion | Renal |
| Identifiers | |
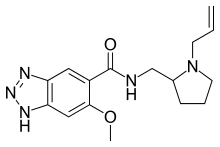
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.056.082 |
| Chemical and physical data | |
| Formula | C16H21N5O2 |
| Molar mass | 315.377 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Alizapride (Litican, Plitican, Superan, Vergentan) is a dopamine antagonist with prokinetic and antiemetic effects used in the treatment of nausea and vomiting, including postoperative nausea and vomiting. It is structurally related to metoclopramide and other benzamides.[1]
References
- ↑ Ballatori E, Roila F (September 2003). "Impact of nausea and vomiting on quality of life in cancer patients during chemotherapy". Health and Quality of Life Outcomes. 1: 46. doi:10.1186/1477-7525-1-46. PMC 212194. PMID 14521717.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.