Roxindole
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
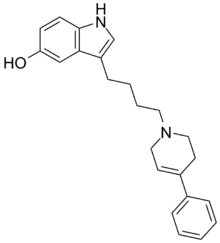
| Formula | C23H26N2O |
| Molar mass | 346.474 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
Roxindole (EMD-49,980) is a dopaminergic and serotonergic drug which was originally developed by Merck KGaA for the treatment of schizophrenia.[1][2][3] In clinical trials its antipsychotic efficacy was only modest but it was unexpectedly found to produce potent and rapid antidepressant and anxiolytic effects.[2][3] As a result, roxindole was further researched for the treatment of depression instead.[1][4] It has also been investigated as a therapy for Parkinson's disease and prolactinoma.[5][6]
Roxindole acts as an agonist at the following receptors:[7][8]
- D2 receptor (pKi = 8.55)
- D3 receptor (pKi = 8.93)
- D4 receptor (pKi = 8.23)
- 5-HT1A receptor (pKi = 9.42)
At D2 and possibly D3 receptors roxindole is a partial agonist with preferential actions at autoreceptors and has been touted as a 'selective' autoreceptor agonist, hence the justification of its application as an antipsychotic.[9][10] Weaker activity at the serotonin 1B and 1D receptors has been seen.[11] It is also a serotonin reuptake inhibitor (IC50 = 1.4 nM) and has been reported to act as a 5-HT2A receptor antagonist as well.[8][9][10][12]
References
- 1 2 Gründer G, Wetzel H, Hammes E, Benkert O (1993). "Roxindole, a dopamine autoreceptor agonist, in the treatment of major depression". Psychopharmacology. 111 (1): 123–6. doi:10.1007/BF02257418. PMID 7870927. S2CID 8154586.
- 1 2 Klimke A, Klieser E (July 1991). "Antipsychotic efficacy of the dopaminergic autoreceptor agonist EMD 49980 (Roxindol). Results of an open clinical study". Pharmacopsychiatry. 24 (4): 107–12. doi:10.1055/s-2007-1014451. PMID 1684439.
- 1 2 Kasper S, Fuger J, Zinner HJ, Bäuml J, Möller HJ (March 1992). "Early clinical results with the neuroleptic roxindole (EMD 49,980) in the treatment of schizophrenia--an open study". European Neuropsychopharmacology. 2 (1): 91–5. doi:10.1016/0924-977X(92)90041-6. PMID 1353388. S2CID 21861347.
- ↑ Maj J, Kolodziejczyk K, Rogóz Z, Skuza G (1996). "Roxindole, a potential antidepressant. I. Effect on the dopamine system". Journal of Neural Transmission. 103 (5): 627–41. doi:10.1007/bf01273159. PMID 8811507. S2CID 33701841.
- ↑ Bravi D, Davis TL, Mouradian MM, Chase TN (April 1993). "Treatment of Parkinson's disease with the partial dopamine agonist EMD 49980". Movement Disorders. 8 (2): 195–7. doi:10.1002/mds.870080214. PMID 8097280. S2CID 23676084.
- ↑ Jaspers C, Benker G, Reinwein D (June 1994). "Treatment of prolactinoma patients with the new non-ergot dopamine agonist roxindol: first results". The Clinical Investigator. 72 (6): 451–6. doi:10.1007/bf00180520. PMID 7950157. S2CID 532184.
- ↑ Newman-Tancredi A, Cussac D, Audinot V, Millan MJ (June 1999). "Actions of roxindole at recombinant human dopamine D2, D3 and D4 and serotonin 5-HT1A, 5-HT1B and 5-HT1D receptors". Naunyn-Schmiedeberg's Archives of Pharmacology. 359 (6): 447–53. doi:10.1007/pl00005374. PMID 10431754. S2CID 19721911. Archived from the original on 2013-02-11.
- 1 2 Heinrich T, Böttcher H, Bartoszyk GD, Greiner HE, Seyfried CA, Van Amsterdam C (September 2004). "Indolebutylamines as selective 5-HT(1A) agonists". Journal of Medicinal Chemistry. 47 (19): 4677–83. doi:10.1021/jm040792y. PMID 15341483.
- 1 2 Seyfried CA, Greiner HE, Haase AF (January 1989). "Biochemical and functional studies on EMD 49,980: a potent, selectively presynaptic D-2 dopamine agonist with actions on serotonin systems". European Journal of Pharmacology. 160 (1): 31–41. doi:10.1016/0014-2999(89)90651-1. PMID 2565817.
- 1 2 Bartoszyk GD, Harting J, Minck KO (January 1996). "Roxindole: psychopharmacological profile of a dopamine D2 autoreceptor agonist". The Journal of Pharmacology and Experimental Therapeutics. 276 (1): 41–8. PMID 8558454.
- ↑ Newman-Tancredi, A.; Cussac, D.; Audinot, V.; Millan, M. J. (Jun 1999). "Actions of roxindole at recombinant human dopamine D2, D3 and D4 and serotonin 5-HT1A, 5-HT1B and 5-HT1D receptors". Naunyn-Schmiedeberg's Archives of Pharmacology. 359 (6): 447–453. doi:10.1007/pl00005374. ISSN 0028-1298. PMID 10431754. S2CID 19721911.
- ↑ Maj J, Kołodziejczyk K, Rogóz Z, Skuza G (March 1997). "Roxindole, a dopamine autoreceptor agonist with a potential antidepressant activity. II. Effects on the 5-hydroxytryptamine system". Pharmacopsychiatry. 30 (2): 55–61. doi:10.1055/s-2007-979483. PMID 9131725.
| 5-HT1AR agonists | |
|---|---|
| GABAAR PAMs |
|
| Gabapentinoids (α2δ VDCC blockers) | |
| Antidepressants |
|
| Sympatholytics (Antiadrenergics) |
|
| Others | |
| |
| Typical |
|
|---|---|
| Disputed | |
| Atypical |
|
| Others |
|
| |
| Dopaminergics |
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anticholinergics | |||||||||||
| Others |
| ||||||||||
| |||||||||||
| D1-like |
| ||||||
|---|---|---|---|---|---|---|---|
| D2-like |
| ||||||
| |||||||
| DAT (DRIs) |
| ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NET (NRIs) |
| ||||||||||||||
| SERT (SRIs) |
| ||||||||||||||
| VMATs |
| ||||||||||||||
| Others |
| ||||||||||||||
See also: Receptor/signaling modulators • Monoamine releasing agents • Adrenergics • Dopaminergics • Serotonergics • Monoamine metabolism modulators • Monoamine neurotoxins | |||||||||||||||
Serotonin receptor modulators | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||