Mesocarb
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | hepatic |
| Excretion | renal |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
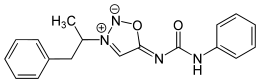
| Formula | C18H18N4O2 |
| Molar mass | 322.368 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Mesocarb (brand names Sidnocarb, Sydnocarb) is a drug that is currently being developed for Parkinson's disease.[1]
The drug was originally developed in the USSR in the 1970s [2][3] for a variety of indications including asthenia, apathy, adynamia and some clinical aspects of depression and schizophrenia.[4][5] Mesocarb was used for counteracting the sedative effects of benzodiazepine drugs,[6] increasing workload capacity and cardiovascular function,[7] treatment of ADHD and hyperactivity in children,[8][9] as a nootropic,[10] and as a drug to enhance resistance to extremely cold temperatures.[11][12] It is also listed as having antidepressant and anticonvulsant properties.
The drug has been found to act as a selective dopamine reuptake inhibitor by blocking the actions of the dopamine transporter (DAT),[13][14] and lacks the dopamine release characteristic of stimulants such as dextroamphetamine.[15][16][17] It was the most selective DAT inhibitor amongst an array of other DAT inhibitors to which it was compared.[14]
Mesocarb was sold in Russia as 5 milligram tablets under the brand name Sydnocarb. Hydroxylated metabolites can be detected in urine for up to 10 days after consumption.[18]
The drug is almost unknown in the western world and is neither used in medicine or studied scientifically to any great extent outside Russia and other countries in the former Soviet Union. It has however been added to the list of drugs under international control and is a scheduled substance in most countries, despite its multiple therapeutic applications and reported lack of significant abuse potential.[19]
Mesocarb had erroneously been referred to as a prodrug of amphetamine[20] but this was based on older literature that relied on gas chromatography as an analytical method. Subsequently, with the advent of mass spectroscopy, it has been shown that presence of amphetamine in prior studies was an artifact of gas chromatography method.[21] More recent studies using mass spectroscopy show that negligible levels of amphetamine are released from mesocarb metabolism.[18]
Chemistry
Mesocarb is a mesoionic sydnone imine. It has the amphetamine-backbone present, except that the RN has a complicated imine side-chain present.
Preparation
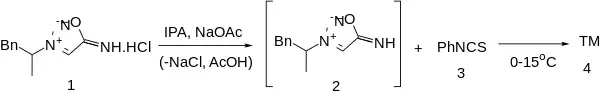
References
- ↑ "Melior Discovery Announces Spinout of Melior Pharmaceuticals II, LLC". 10 May 2016.
- ↑ GB Patent 1262830 - NOVEL SYDNONIMINE DERIVATIVE
- ↑ Anokhina IP, Zabrodin GD, Svirinovskiĭ I (1974). "[Characteristics of the central action of sidnocarb]" [Characteristics of the central action of sidnocarb]. Zhurnal Nevropatologii I Psikhiatrii Imeni S.S. Korsakova (in Russian). 74 (4): 594–602. PMID 4825943.
- ↑ Rudenko GM, Altshuler RA (1979). "Peculiarities of clinical activity and pharmacokinetics of sydnocarb (sydnocarbum), an original psychostimulant". Agressologie. 20 (D): 265–70. PMID 45391.
- ↑ Witkin JM, Savtchenko N, Mashkovsky M, Beekman M, Munzar P, Gasior M, et al. (March 1999). "Behavioral, toxic, and neurochemical effects of sydnocarb, a novel psychomotor stimulant: comparisons with methamphetamine". The Journal of Pharmacology and Experimental Therapeutics. 288 (3): 1298–310. PMID 10027871.
- ↑ Valueva LN, Tozhanova NM (1982). "[Sidnocarb correction of the adverse effects of benzodiazepine tranquilizers]" [Sidnocarb correction of the adverse effects of benzodiazepine tranquilizers]. Zhurnal Nevropatologii I Psikhiatrii Imeni S.S. Korsakova (in Russian). 82 (8): 92–7. PMID 6127851.
- ↑ Vinar O, Klein DF, Potter WZ, Gause EM (December 1991). "A survey of psychotropic medications not available in the United States". Neuropsychopharmacology. 5 (4): 201–17. PMID 1804161.
- ↑ Turova NF, Misionzhnik EI, Ermolina LA, Aziavchik AV, Krasov VA (1988). "[Excretion of monoamines, their precursors and metabolites in the hyperactivity syndrome in mentally defective children]" [Excretion of monoamines, their precursors and metabolites in the hyperactivity syndrome in mentally defective children]. Voprosy Meditsinskoi Khimii (in Russian). 34 (1): 47–50. PMID 3369126.
- ↑ Krasov VA (1988). "[Sidnocarb treatment of young schoolchildren with the hyperdynamic syndrome]" [Sidnocarb treatment of young schoolchildren with the hyperdynamic syndrome]. Zhurnal Nevropatologii I Psikhiatrii Imeni S.S. Korsakova (in Russian). 88 (8): 97–101. PMID 3195293.
- ↑ Ganiev MM, Kharlamov AN, Raevskiĭ KS, Guseĭnov DI (October 1987). "[Effect of sidnocarb on learning and memory]" [Effect of sidnocarb on learning and memory]. Biulleten' Eksperimental'noi Biologii I Meditsiny (in Russian). 104 (10): 453–4. PMID 3676468.
- ↑ Barer AS, Lakota NG, Ostrovskaia GZ, Shashkov VS (Nov–Dec 1988). "[Pharmacologic correction of the effect of cold on man]" [Pharmacologic correction of the effect of cold on man]. Kosmicheskaia Biologiia I Aviakosmicheskaia Meditsina (in Russian). 22 (6): 66–73. PMID 2906380.
- ↑ Levina MN, Badyshtov BA, Gan'shina TS (2006). "[Thermoprotector properties of a combination of sydnocarb with ladasten]" [Thermoprotector properties of a combination of sydnocarb with ladasten]. Eksperimental'naia i Klinicheskaia Farmakologiia (in Russian). 69 (1): 71–3. PMID 16579065.
- ↑ Erdö SL, Kiss B, Rosdy B (1981). "Inhibition of dopamine uptake by a new psychostimulant mesocarb (Sydnocarb)". Polish Journal of Pharmacology and Pharmacy. 33 (2): 141–7. PMID 7312716.
- 1 2 Gruner JA, Mathiasen JR, Flood DG, Gasior M (May 2011). "Characterization of pharmacological and wake-promoting properties of the dopaminergic stimulant sydnocarb in rats". The Journal of Pharmacology and Experimental Therapeutics. 337 (2): 380–90. doi:10.1124/jpet.111.178947. PMID 21300706. S2CID 9985668.
- ↑ Afanas'ev II, Anderzhanova EA, Kudrin VS, Rayevsky KS (2001). "Effects of amphetamine and sydnocarb on dopamine release and free radical generation in rat striatum". Pharmacology, Biochemistry, and Behavior. 69 (3–4): 653–8. doi:10.1016/S0091-3057(01)00574-3. PMID 11509228. S2CID 32739707.
- ↑ Anderzhanova EA, Afanas'ev II, Kudrin VS, Rayevsky KS (September 2000). "Effect of d-amphetamine and sydnocarb on the extracellular level of dopamine, 3,4-dihydroxyphenylacetic acid, and hydroxyl radicals generation in rat striatum". Annals of the New York Academy of Sciences. 914: 137–45. doi:10.1111/j.1749-6632.2000.tb05191.x. PMID 11085316. S2CID 12326076.
- ↑ Gainetdinov RR, Sotnikova TD, Grekhova TV, Rayevsky KS (December 1997). "Effects of a psychostimulant drug sydnocarb on rat brain dopaminergic transmission in vivo". European Journal of Pharmacology. 340 (1): 53–8. doi:10.1016/S0014-2999(97)01407-6. PMID 9527506.
- 1 2 Shpak AV, Appolonova SA, Semenov VA (January 2005). "Validation of liquid chromatography-electrospray ionization ion trap mass spectrometry method for the determination of mesocarb in human plasma and urine". Journal of Chromatographic Science. 43 (1): 11–21. doi:10.1093/chromsci/43.1.11. PMID 15808002.
- ↑ Rudenko GM, Altshuler RA (1978). "[Experimental and clinical study of Sydnocarb]". Hung Pharmacotherapy (in Russian). 124: 150–4.
- ↑ Dettmeyer R, Verhoff MA, Schütz HF (9 October 2013). Forensic Medicine: Fundamentals and Perspectives. Springer Science & Business Media. pp. 519–. ISBN 978-3-642-38818-7.
- ↑ Appolonova SA, Shpak AV, Semenov VA (February 2004). "Liquid chromatography-electrospray ionization ion trap mass spectrometry for analysis of mesocarb and its metabolites in human urine". Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences. 800 (1–2): 281–9. doi:10.1016/j.jchromb.2003.10.071. PMID 14698267.