Tranylcypromine
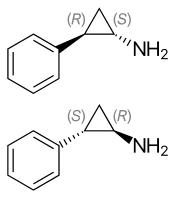 (1S,2R)-(−)-tranylcypromine (top), (1R,2S)-(+)-tranylcypromine (bottom) | |
| Names | |
|---|---|
| Trade names | Parnate, others[1] |
| Other names | trans-2-phenylcyclopropylamine |
IUPAC name
| |
| Clinical data | |
| Drug class | Monoamine oxidase inhibitor (MAOI)[2] |
| Main uses | Major depressive disorder[2] |
| Side effects | Trouble sleeping, nausea, dry mouth, fast heart rate, sexual dysfunction[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth |
| Onset of action | Up to 3 weeks[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682088 |
| Legal | |
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | 50%[3] |
| Metabolism | Liver[4][5] |
| Elimination half-life | 2.5 hours[3] |
| Excretion | Urine, feces[3] |
| Chemical and physical data | |
| Formula | C9H11N |
| Molar mass | 133.194 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
SMILES
| |
InChI
| |
Tranylcypromine, sold under the trade name Parnate among others, is a medication used to treat major depressive disorder.[2] It; however, is not used as a first line treatment.[2] It is taken by mouth.[2] Onset of effects may require up to 3 weeks.[2]
Common side effects may include trouble sleeping, nausea, dry mouth, fast heart rate, and sexual dysfunction.[2] Other side effect may include high blood pressure, mania, serotonin syndrome, and an increased risk of suicide in those under the age of 25.[2] It is a monoamine oxidase inhibitor (MAOI) and believed to work by increasing serotonin and norepinephrine.[2]
Tranylcypromine was approved for medical use in the United States in 1961.[2] It is available as a generic medication.[6] In the United Kingdom it costs the NHS about £370 at 10 mg per day for a month as of 2021.[6] This amount in the United States costs about 22 USD.[7]
Medical uses
Tranylcypromine is used to treat major depressive disorder, including atypical depression, especially when there is an anxiety component, typically as a second-line treatment.[8] It is also used in depression that is not responsive to reuptake inhibitor antidepressants, such as the SSRIs, TCAs, or bupropion.[9]
Dosage
It is generally used at a dose of 10 to 30 mg in two doses per day.[6] The maximum dose is 60 mg per day.[2]
Contraindications
Contraindications include:[8][9][10]
- Porphyria
- Cardiovascular or cerebrovascular disease
- Pheochromocytoma
- Tyramine, found in several foods, is metabolized by MAO. Ingestion and absorption of tyramine causes extensive release of norepinephrine, which can rapidly increase blood pressure to the point of causing hypertensive crisis.
- Concomitant use of serotonin-enhancing drugs, including SSRIs, serotonergic TCAs, dextromethorphan, and meperidine may cause serotonin syndrome.
- Concomitant use of MRAs, including fenfluramine, amphetamine, and pseudoephedrine may cause toxicity via serotonin syndrome or hypertensive crisis.
- L-DOPA given without carbidopa may cause hypertensive crisis.
Dietary restrictions
Tyramine is a common component in many foods, and is normally rapidly metabolized by MAO-A. Individuals not taking MAOIs may consume at least 2 grams of tyramine in a meal and not experience an increase in blood pressure, whereas those taking MAOIs such as tranylcypromine may experience a sharp increase in blood pressure following consumption of as little as 10 mg of tyramine, which can lead to hypertensive crisis.[11][10]
Foods containing tyramine include aged cheeses, cured meats, tofu and certain red wines. Some, such as yeast extracts, contain enough tyramine to be potentially fatal in a single serving. Spoiled food is also likely to contain dangerous levels of tyramine.[8]
Side effects
Rates of side effects[12]
Very common (>10% incidence) adverse effects include:
- Dizziness secondary to orthostatic hypotension (17%)
Common (1-10% incidence) adverse effects include:
- Tachycardia (5-10%)
- Hypomania (7%)
- Paresthesia (5%)
- Weight loss (2%)
- Confusion (2%)
- Dry mouth (2%)
- Sexual function disorders (2%)
- Hypertension (1–2 hours after ingestion) (2%)
- Rash (2%)
- Urinary retention (2%)
Other (unknown incidence) adverse effects include:
- Increased/decreased appetite
- Blood dyscrasias
- Chest pain
- Diarrhea
- Edema
- Hallucinations
- Hyperreflexia
- Insomnia
- Jaundice
- Leg cramps
- Myalgia
- Palpitations
- Sensation of cold
- Suicidal ideation
- Tremor
Of note, there has not been found to be a correlation between sex and age below 65 regarding incidence of adverse effects.[12]
Tranylcypromine is not associated with weight gain and has a low risk for hepatotoxicity compared to the hydrazine MAOIs.[12][9]
It is generally recommended that MAOIs be discontinued prior to anesthesia; however, this creates a risk of recurrent depression. In a retrospective observational cohort study, patients on tranylcypromine undergoing general anesthesia had a lower incidence of intraoperative hypotension, while there was no difference between patients not taking an MAOI regarding intraoperative incidence of bradycardia, tachycardia, or hypertension.[13] The use of indirect sympathomimetic drugs or drugs affecting serotonin reuptake, such as meperidine or dextromethorphan poses a risk for hypertension and serotonin syndrome respectively; alternative agents are recommended.[14][15] Other studies have come to similar conclusions.[12] Pharmacokinetic interactions with anesthetics are unlikely, given that tranylcypromine is a high-affinity substrate for CYP2A6 and does not inhibit CYP enzymes at therapeutic concentrations.[11]
Tranylcypromine abuse has been reported at doses ranging from 120–600 mg per day.[8][16][12] It is thought that higher doses have more amphetamine-like effects and abuse is promoted by the fast onset and short half-life of tranylcypromine.[12]
Cases of suicidal ideation and suicidal behaviours have been reported during tranylcypromine therapy or early after treatment discontinuation.[8]
Symptoms of tranylcypromine overdose are generally more intense manifestations of its usual effects.[8]
Interactions
In addition to contraindicated concomitant medications, tranylcypromine inhibits CYP2A6, which may reduce the metabolism and increase the toxicity of substrates of this enzyme, such as:[10]
- Dexmedetomidine
- nicotine
- TSNAs (found in cured tobacco products, including cigarettes)
- Valproate
Norepinephrine reuptake inhibitors prevent neuronal uptake of tyramine and may reduce its pressor effects.[10]
Pharmacology
Pharmacodynamics
Tranylcypromine acts as a nonselective and irreversible inhibitor of monoamine oxidase.[3] Regarding the isoforms of monoamine oxidase, it shows slight preference for the MAOB isoenzyme over MAOA.[11] This leads to an increase in the availability of monoamines, such as serotonin, norepinephrine, and dopamine, as well as a marked increase in the availability of trace amines, such as tryptamine, octopamine, and phenethylamine.[11][10] The clinical relevance of increased trace amine availability is unclear.
It may also act as a norepinephrine reuptake inhibitor at higher therapeutic doses.[11] Compared to amphetamine, tranylcypromine shows low potency as a dopamine releasing agent, with even weaker potency for norepinephrine and serotonin release.[11][10]
Tranylcypromine has also been shown to inhibit the histone demethylase, BHC110/LSD1. Tranylcypromine inhibits this enzyme with an IC50 < 2 μM, thus acting as a small molecule inhibitor of histone demethylation with an effect to derepress the transcriptional activity of BHC110/LSD1 target genes.[17] The clinical relevance of this effect is unknown.
Tranylcypromine has been found to inhibit CYP46A1 at nanomolar concentrations.[18] The clinical relevance of this effect is unknown.

Pharmacokinetics
Tranylcypromine reaches its maximum concentration (tmax) within 1–2 hours.[11] After a 20 mg dose, plasma concentrations reach at most 50-200 ng/mL.[11] While its half-life is only about 2 hours, its pharmacodynamic effects last several days to weeks due to irreversible inhibition of MAO.[11]
Metabolites of tranylcypromine include 4-hydroxytranylcypromine, N-acetyltranylcypromine, and N-acetyl-4-hydroxytranylcypromine, which are less potent MAO inhibitors than tranylcypromine itself.[11] Amphetamine was once thought to be a metabolite of tranylcypromine, but has not been shown to be.[11][20][10]
Tranylcypromine inhibits CYP2A6 at therapeutic concentrations.[10]
Chemistry

Synthesis
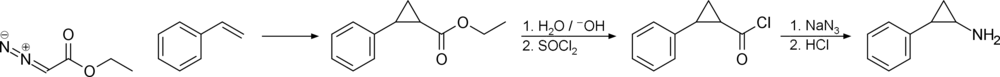
History
Tranylcypromine was originally developed as an analog of amphetamine.[3][11] Although it was first synthesized in 1948,[22] its MAOI action was not discovered until 1959. Precisely because tranylcypromine was not, like isoniazid and iproniazid, a hydrazine derivative, its clinical interest increased enormously, as it was thought it might have a more acceptable therapeutic index than previous MAOIs.[23]
The drug was introduced by Smith, Kline and French in the United Kingdom in 1960, and approved in the United States in 1961.[24] It was withdrawn from the market in February 1964 due to a number of patient deaths involving hypertensive crises with intracranial bleeding. However, it was reintroduced later that year with more limited indications and specific warnings of the risks.[25][11][10]
Research
Tranylcypromine is known to inhibit LSD1, an enzyme that selectively demethylates two lysines found on histone H3.[17][11][26] Genes promoted downstream of LSD1 are involved in cancer cell growth and metastasis, and several tumor cells express high levels of LSD1.[26] Tranylcypromine analogues with more potent and selective LSD1 inhibitory activity are being researched in the potential treatment of cancers.[26][27]
Tranylcypromine may have neuroprotective properties applicable to the treatment of Parkinson's disease, similar to the MAO-B inhibitors selegiline and rasagiline.[28][9] As of 2017, only one clinical trial in Parkinsonian patients has been conducted, which found some improvement initially and only slight worsening of symptoms after a 1.5 year followup.[9]
See also
- Phenelzine
- Amphetamine
- Cibenzoline (also based on cyclopropane)
- Ticagrelor (contains 3',4'-difluoro-tranylcypromine structural motif)
- Tranylcypromine/trifluoperazine
References
- ↑ "Tranylcypromine Uses, Side Effects & Warnings". Drugs.com. Archived from the original on 29 August 2017. Retrieved 16 September 2021.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 "Tranylcypromine Monograph for Professionals". Drugs.com. Archived from the original on 20 January 2021. Retrieved 16 September 2021.
- 1 2 3 4 5 Williams DA (2007). "Antidepressants". In Foye, William O., Lemke, Thomas L., Williams, David A. (eds.). Foye's Principles of Medicinal Chemistry. Hagerstwon, USA: Lippincott Williams & Wilkins. pp. 590–1. ISBN 978-0-7817-6879-5. Archived from the original on 2021-08-29. Retrieved 2021-05-30.
- ↑ "Tranylcypromine". www.drugbank.ca. Archived from the original on 2019-10-23. Retrieved 2019-12-06.
- ↑ Baker GB, Urichuk LJ, McKenna KF, Kennedy SH (June 1999). "Metabolism of monoamine oxidase inhibitors". Cellular and Molecular Neurobiology. 19 (3): 411–26. doi:10.1023/a:1006901900106. PMID 10319194. S2CID 21380176.
- 1 2 3 BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 380. ISBN 978-0-85711-369-6.
{{cite book}}: CS1 maint: date format (link) - ↑ "Tranylcypromine Prices, Coupons & Savings Tips - GoodRx". GoodRx. Retrieved 17 September 2021.
- 1 2 3 4 5 6 UK Electronic medicines compendium. Tranylcypromine Archived 2018-10-17 at the Wayback Machine Llast updated October 28, 2015
- 1 2 3 4 5 Riederer P, Laux G (March 2011). "MAO-inhibitors in Parkinson's Disease". Experimental Neurobiology. 20 (1): 1–17. doi:10.5607/en.2011.20.1.1. PMC 3213739. PMID 22110357.
- 1 2 3 4 5 6 7 8 9 Gillman PK (February 2011). "Advances pertaining to the pharmacology and interactions of irreversible nonselective monoamine oxidase inhibitors". Journal of Clinical Psychopharmacology. 31 (1): 66–74. doi:10.1097/JCP.0b013e31820469ea. PMID 21192146. S2CID 10525989.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Ulrich S, Ricken R, Adli M (August 2017). "Tranylcypromine in mind (Part I): Review of pharmacology". European Neuropsychopharmacology. 27 (8): 697–713. doi:10.1016/j.euroneuro.2017.05.007. PMID 28655495. S2CID 4913721.
- 1 2 3 4 5 6 Ricken R, Ulrich S, Schlattmann P, Adli M (August 2017). "Tranylcypromine in mind (Part II): Review of clinical pharmacology and meta-analysis of controlled studies in depression". European Neuropsychopharmacology. 27 (8): 714–731. doi:10.1016/j.euroneuro.2017.04.003. PMID 28579071. S2CID 30987747.
- ↑ van Haelst IM, van Klei WA, Doodeman HJ, Kalkman CJ, Egberts TC (August 2012). "Antidepressive treatment with monoamine oxidase inhibitors and the occurrence of intraoperative hemodynamic events: a retrospective observational cohort study". The Journal of Clinical Psychiatry. 73 (8): 1103–9. doi:10.4088/JCP.11m07607. PMID 22938842.
- ↑ Smith MS, Muir H, Hall R (February 1996). "Perioperative management of drug therapy, clinical considerations". Drugs. 51 (2): 238–59. doi:10.2165/00003495-199651020-00005. PMID 8808166. S2CID 46972638.
- ↑ Blom-Peters L, Lamy M (1993). "Monoamine oxidase inhibitors and anesthesia: an updated literature review". Acta Anaesthesiologica Belgica. 44 (2): 57–60. PMID 8237297.
- ↑ Le Gassicke J, Ashcroft GW, Eccleston D, Evans JI, Oswald I, Ritson EB (1 April 1965). "The Clinical State, Sleep and Amine Metabolism of a Tranylcypromine ('Parnate') Addict". The British Journal of Psychiatry. 111 (473): 357–364. doi:10.1192/bjp.111.473.357.
- 1 2 Lee MG, Wynder C, Schmidt DM, McCafferty DG, Shiekhattar R (June 2006). "Histone H3 lysine 4 demethylation is a target of nonselective antidepressive medications". Chemistry & Biology. 13 (6): 563–7. doi:10.1016/j.chembiol.2006.05.004. PMID 16793513.
- ↑ Mast N, Charvet C, Pikuleva IA, Stout CD (October 2010). "Structural basis of drug binding to CYP46A1, an enzyme that controls cholesterol turnover in the brain". The Journal of Biological Chemistry. 285 (41): 31783–95. doi:10.1074/jbc.M110.143313. PMC 2951250. PMID 20667828.
- ↑ Gaweska H, Fitzpatrick PF (October 2011). "Structures and Mechanism of the Monoamine Oxidase Family". Biomolecular Concepts. 2 (5): 365–377. doi:10.1515/BMC.2011.030. PMC 3197729. PMID 22022344.
- ↑ Sherry RL, Rauw G, McKenna KF, Paetsch PR, Coutts RT, Baker GB (December 2000). "Failure to detect amphetamine or 1-amino-3-phenylpropane in humans or rats receiving the MAO inhibitor tranylcypromine". Journal of Affective Disorders. 61 (1–2): 23–9. doi:10.1016/s0165-0327(99)00188-3. PMID 11099737.
- ↑ A US patent 4016204 A, Vithal Jagannath Rajadhyaksha, "Method of synthesis of trans-2-phenylcyclopropylamine", published 1977-04-05, assigned to Nelson Research & Development Company
- ↑ Burger A, Yost WL (1948). "Arylcycloalkylamines. I. 2-Phenylcyclopropylamine". Journal of the American Chemical Society. 70 (6): 2198–2201. doi:10.1021/ja01186a062.
- ↑ López-Muñoz F, Alamo C (2009). "Monoaminergic neurotransmission: the history of the discovery of antidepressants from 1950s until today". Current Pharmaceutical Design. 15 (14): 1563–86. doi:10.2174/138161209788168001. PMID 19442174.
- ↑ Shorter E (2009). Before Prozac: the troubled history of mood disorders in psychiatry. Oxford [Oxfordshire]: Oxford University Press. ISBN 978-0-19-536874-1. Archived from the original on 2021-08-29. Retrieved 2021-05-30.
- ↑ Atchley DW (September 1964). "Reevaluation of Tranylcypromine Sulfate(Parnate Sulfate)". JAMA. 189 (10): 763–4. doi:10.1001/jama.1964.03070100057011. PMID 14174054.
- 1 2 3 Zheng YC, Yu B, Jiang GZ, Feng XJ, He PX, Chu XY, et al. (2016). "Irreversible LSD1 Inhibitors: Application of Tranylcypromine and Its Derivatives in Cancer Treatment". Current Topics in Medicinal Chemistry. 16 (19): 2179–88. doi:10.2174/1568026616666160216154042. PMID 26881714.
- ↑ Przespolewski A, Wang ES (July 2016). "Inhibitors of LSD1 as a potential therapy for acute myeloid leukemia". Expert Opinion on Investigational Drugs. 25 (7): 771–80. doi:10.1080/13543784.2016.1175432. PMID 27077938. S2CID 20858344.
- ↑ Al-Nuaimi SK, Mackenzie EM, Baker GB (November 2012). "Monoamine oxidase inhibitors and neuroprotection: a review". American Journal of Therapeutics. 19 (6): 436–48. doi:10.1097/MJT.0b013e31825b9eb5. PMID 22960850.
External links
| Identifiers: |
|---|