Ifetroban
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
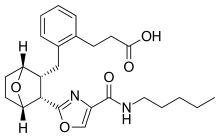
| Formula | C25H32N2O5 |
| Molar mass | 440.540 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
Ifetroban is a potent and selective thromboxane receptor antagonist.[1] It has been studied in animal models for the treatment of myocardial ischemia, hypertension, stroke, thrombosis, cardiomyopathy, and for its effects on platelets.[2][3]
Synthesis
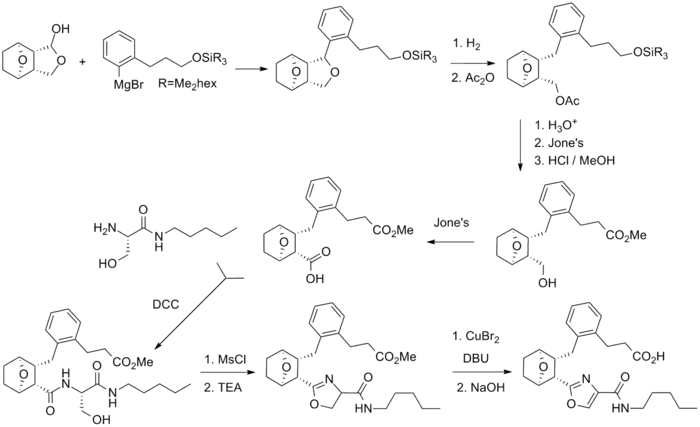
Ifetroban synthesis: Drugs Future (c.f. Lednicer book 6).
References
- ↑ Rosenfeld, Louis; Grover, Gary J.; Stier, Charles T. (2006). "Ifetroban Sodium: An Effective TxA2/PGH2 Receptor Antagonist". Cardiovascular Drug Reviews. 19 (2): 97–115. doi:10.1111/j.1527-3466.2001.tb00058.x. PMID 11484065.
- ↑ Dockens RC, Santone KS, Mitroka JG, Morrison RA, Jemal M, Greene DS, Barbhaiya RH (August 2000). "Disposition of radiolabeled ifetroban in rats, dogs, monkeys, and humans" (PDF). Drug Metabolism and Disposition. 28 (8): 973–80. PMID 10901709.
- ↑ West, James D.; Galindo, Cristi L.; Kim, Kyungsoo; Shin, John Jonghyun; Atkinson, James B.; MacIas‐Perez, Ines; Pavliv, Leo; Knollmann, Bjorn C.; Soslow, Jonathan H.; Markham, Larry W.; Carrier, Erica J. (2019). "Antagonism of the Thromboxane‐Prostanoid Receptor as a Potential Therapy for Cardiomyopathy of Muscular Dystrophy". Journal of the American Heart Association. 8 (21): e011902. doi:10.1161/JAHA.118.011902. PMC 6898850. PMID 31662020.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.