Dexibuprofen
 | |
-ibuprofen-3D-balls.png.webp) | |
| Clinical data | |
|---|---|
| Trade names | Seractil, Deltaran, Ibusoft, Monactil |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.106.960 |
| Chemical and physical data | |
| Formula | C13H18O2 |
| Molar mass | 206.285 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Dexibuprofen is a nonsteroidal anti-inflammatory drug (NSAID).
It is the active dextrorotatory enantiomer of ibuprofen.[1] Most ibuprofen formulations contain a racemic mixture of both isomers.
Chemistry
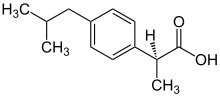
Basically Dexibuprofen is a chiral switch of racemic ibuprofen. The chiral carbon in dexibuprofen is assigned an absolute configuration, (S), as per Cahn-Ingold-prelog rule.[2][3]
Pharmacology
Ibuprofen, is an α-arylpropionic acid, used largely in the treatment of rheumatoid arthritis and widely used over-the counter drug for headache and minor pains. This drug has a chiral center and exists as a pair of enantiomers. The (S)- ibuprofen, the eutomer, is responsible for the desired therapeutic effect. Interestingly, the inactive (R)-enantiomer, the distomer, undergoes a unidirectional chiral inversion to give the active (S)-enantiomer, the former acting as a pro-drug for the latter.[4][5][6][7] That is, when the ibuprofen is administered as a racemate the distomer is converted in vivo into the eutomer while the latter is unaffected. This chiral inversion may lead to accumulation of one of the enantiomers leading to toxicity. The risk of side-effect can be avoided by the use of pure (S)-enantiomer.[8]
See also
- Chiral switch
- Enantiopure drug
- Chirality
- Eudysmic ratio
References
- ↑ Hardikar MS (September 2008). "Chiral non-steroidal anti-inflammatory drugs--a review". Journal of the Indian Medical Association. 106 (9): 615–8, 622, 624. PMID 19552094.
- ↑ Cahn, R. S.; Ingold, Christopher; Prelog, V. (1966). "Specification of Molecular Chirality". Angewandte Chemie International Edition in English. 5 (4): 385–415. doi:10.1002/anie.196603851. ISSN 0570-0833.
- ↑ Cahn, R. S.; Ingold, C. K.; Prelog, V. (1956). "The specification of asymmetric configuration in organic chemistry". Experientia. 12 (3): 81–94. doi:10.1007/bf02157171. ISSN 0014-4754.
- ↑ Caldwell J, Hutt AJ, Fournel-Gigleux S (January 1988). "The metabolic chiral inversion and dispositional enantioselectivity of the 2-arylpropionic acids and their biological consequences". Biochemical Pharmacology. 37 (1): 105–14. doi:10.1016/0006-2952(88)90762-9. PMID 3276314.
- ↑ Hutt AJ, Caldwell J (November 1983). "The metabolic chiral inversion of 2-arylpropionic acids--a novel route with pharmacological consequences". The Journal of Pharmacy and Pharmacology. 35 (11): 693–704. doi:10.1111/j.2042-7158.1983.tb02874.x. PMID 6139449.
- ↑ Adams SS, Bresloff P, Mason CG (March 1976). "Pharmacological differences between the optical isomers of ibuprofen: evidence for metabolic inversion of the (-)-isomer". The Journal of Pharmacy and Pharmacology. 28 (3): 256–7. doi:10.1111/j.2042-7158.1976.tb04144.x. PMID 6706.
- ↑ Hao H, Wang G, Sun J (2005). "Enantioselective pharmacokinetics of ibuprofen and involved mechanisms". Drug Metabolism Reviews. 37 (1): 215–34. doi:10.1081/dmr-200047999. PMID 15747501.
- ↑ Simonyi M (1984). "On chiral drug action". Medicinal Research Reviews. 4 (3): 359–413. doi:10.1002/med.2610040304. PMID 6087043.