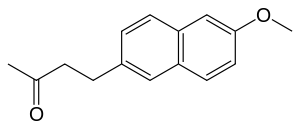
Nabumetone
 | |
| Names | |
|---|---|
| Trade names | Relafen, Relifex, Gambaran, others |
IUPAC name
| |
| Clinical data | |
| Drug class | NSAID[1] |
| Main uses | Inflammation[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Routes of use | By mouth |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a692022 |
| Legal | |
| Legal status |
|
| Pharmacokinetics | |
| Protein binding | > 99% (active metabolite) |
| Metabolism | Liver, to active metabolite 6-methoxy-2-naphthylacetic acid; 6-MNA |
| Elimination half-life | 23 hours (active metabolite) |
| Excretion | Kidney |
| Chemical and physical data | |
| Formula | C15H16O2 |
| Molar mass | 228.291 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Nabumetone is a nonsteroidal anti-inflammatory drug (NSAID), used to treat inflammation in conditions such as osteoarthritis and rheumatoid arthritis.[1] Use is recommended for a short a duration as possible.[2]
Common side effects include abdominal pain, constipation, dizziness, swelling, headache, rash, and ringing in the ears.[1] Other side effects may include kidney problems, heart attacks, gastrointestinal bleeding, high blood pressure, anaphylaxis, and heart failure.[1] Use during the later part of pregnancy may harm the baby.[3] It works by blocking COX-1 and COX-2.[1]
Nabumetone was approved for medical use in the United States in 1991.[1] In the United Kingdom 4 weeks of treatment costs the NHS about £7.[3] This amount in the United States costs about 26 USD.[4]
Medical uses
Similar in action to other NSAIDs, Nabumetone is used to treat pain and inflammation.
Dosage
It is take at a dose of 0.5 to 1 gram once to twice per day.[3]
Side effects
It has been shown to have a slightly lower risk of gastrointestinal side effects than most other non-selective NSAIDs since it is a non-acidic prodrug which is then metabolized to its active 6MNA (6-methoxy-2-naphthylacetic acid) form.
Side effects include: Bloody or black, tarry stools; change in color, frequency, or amount of urine; chest pain; shortness of breath; coughing up blood; pale stools; numbness; weakness; flu-like symptoms; leg pain; vision problems; speech problems; problems walking; weight gain; stomach pain; cold sweat; skin rash; blisters; headache; swelling; bleeding; bruising; vomiting blood; jaundice; diarrhea; constipation; dizziness; indigestion; gas; nausea; and ringing in the ears.[5]
In October 2020, the U.S. Food and Drug Administration (FDA) required the drug label to be updated for all nonsteroidal anti-inflammatory medications to describe the risk of kidney problems in unborn babies that result in low amniotic fluid.[6][7] They recommend avoiding NSAIDs in pregnant women at 20 weeks or later in pregnancy.[6][7]
Mechanism of action
There are two known polymorphs of the compound.[9]
Society and culture
Cost
The medication has a cost in the U.S. of $21 (USD) for 20 tablets (750 mg)[10]
.svg.png.webp) Nabumetone costs (US)
Nabumetone costs (US).svg.png.webp) Nabumetone prescriptions (US)
Nabumetone prescriptions (US)
References
- 1 2 3 4 5 6 7 "Nabumetone Monograph for Professionals". Drugs.com. Archived from the original on 7 March 2021. Retrieved 11 November 2021.
- ↑ "DailyMed - NABUMETONE tablet, film coated". dailymed.nlm.nih.gov. Archived from the original on 13 November 2021. Retrieved 11 November 2021.
- 1 2 3 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 1194. ISBN 978-0857114105.
- ↑ "Nabumetone Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 21 December 2019. Retrieved 11 November 2021.
- ↑ "Relafen (Nabumetone): Side Effects, Interactions, Warning, Dosage & Uses". RxList. Archived from the original on 2018-03-10. Retrieved 2018-03-09.
- 1 2 "FDA Warns that Using a Type of Pain and Fever Medication in Second Half of Pregnancy Could Lead to Complications". U.S. Food and Drug Administration (FDA) (Press release). 15 October 2020. Archived from the original on 16 October 2020. Retrieved 15 October 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - 1 2 "NSAIDs may cause rare kidney problems in unborn babies". U.S. Food and Drug Administration. 21 July 2017. Archived from the original on 17 October 2020. Retrieved 15 October 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ Varfaj, F.; Zulkifli, S. N. A.; Park, H.-G.; Challinor, V. L.; De Voss, J. J.; Ortiz de Montellano, P. R. (2014-02-28). "Carbon-Carbon Bond Cleavage in Activation of the Prodrug Nabumetone". Drug Metabolism and Disposition. 42 (5): 828–838. doi:10.1124/dmd.114.056903. ISSN 1521-009X. PMC 3989788. PMID 24584631.
- ↑ Price, C P; Grzesiak, A L; Lang, M; Matzger, A J (2002). "Polymorphism of Nabumetone". Crystal Growth & Design. 2 (6): 501–503. doi:10.1021/cg0255568.
- ↑ "Nabumetone Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 21 December 2019. Retrieved 5 April 2021.
External links
| Identifiers: |
|---|